Overview
In this lab you will learn how to locate elements of symmetry in molecules and how to demonstrate the results of symmetry operations.
1) Make following molecules (H2O, BF3, CH4, PCl5, C2H6, SF6)using balls and sticks given.
Draw them below:
Background
Molecular symmetry is of great value in understanding molecular bonding and spectroscopy. The symmetry of a molecule is expressed as a collection of symmetry operations or elements of symmetry. A molecule with a particular set of symmetry elements is said to belong to a particular point group. Molecular orbitals and vibrations within molecules also have symmetry properties. Symmetry operations are manipulations of a molecule which result in indistinguishable representations of the molecule; that is, a molecule looks no different before and following a symmetry operation. The operation may exchange two identical atoms, or it may leave one or more atoms unmoved.
The symmetry operations are as follows:
Identity (E): The identity is a trivial symmetry operation which involves doing nothing to the molecule. It is mentioned here only for the sake of completeness. All molecules possess an identity and, in some molecules, the identity is the only element of symmetry.
2) Write how identity (E) is obtained for BF3,
CH4, PCl5, C2H6, SF6 .
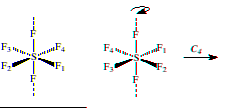
Axis of Rotation (Cn): An n-fold axis of rotation is a rotation about an axis by 360°/n. Often, but not always, rotation axes correspond to bond axes. For example, SF6 possesses a C4 axis which passes through opposing S-F bonds. Rotation by 90° about the axis rotates each fluorine atom perpendicular to the axis into a position previously occupied by another fluorine atom.
3) Draw a model depicting C4 axises of SF6.

SF6 also possesses 3 C3 axes of rotation which do not pass through bonds. In the following representation, the C3 axis is perpendicular to the plane of the paper and passes through the sulfur atom.
4) Draw a model depicting C3 axises of SF6.

Rotate a molecule
5) Make a drawing of your model depicting mirror planes.
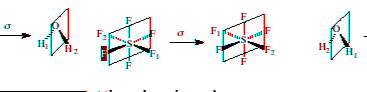
Reflect through a mirror plane
Improper Rotation or rotation-reflection (Sn): An improper rotation is the result of two operations in sequence; rotation by 360°/n, followed by reflection through a mirror plane perpendicular to the axis of rotation. An example of an improper axis of rotation is shown below.
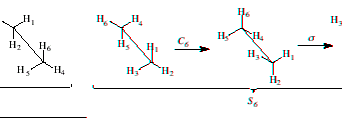
Execute and demonstrate an improper rotation
Follow the procedures for rotation and reflection in sequence.
6) To demonstrate the example above, label carbon and hydrogen atoms, write the operations for each step
7) Label carbon and hydrogen atoms and write two operations (C3 and s) of on the diagram below
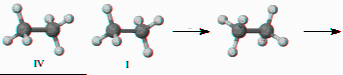
Inversion (i): An inversion through the center of a molecule can usually be accomplished by a rotation by 180° followed by reflection through a plane (Mirror operation) perpendicular to the rotation. Note that this is identical to an S2 as in the example below (rotation by 180° about the C-C axis followed by reflection through a plane perpendicular to the C-C axis).
8) Label carbon and hydrogen atoms and write operation (i) of on the diagram below
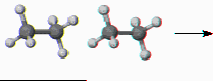
A planar molecule, an inversion is simply accomplished via rotation by 180° about an axis perpendicular to the plane since subsequent reflection through the plane leaves the molecule unchanged.
8) Label boron and fluorine atoms in BF3 and write operation (i) of on a diagram.
Point Groups
Two molecules are in the same point group if they possess the same elements of symmetry. The first step in a group theory analysis of a molecule is to determine the point group of the molecule. There is a systematic way of naming most point groups
9) Using the chart given blow assign the point group to each of the following molecules

The top row and first column consist of the symmetry operations and irreducible representations respectively. The table elements are the characters. The final two columns show the first and second order combinations of Cartesian coordinates. Infinitesimal rotations are listed as Ix, Iy, and Iz.
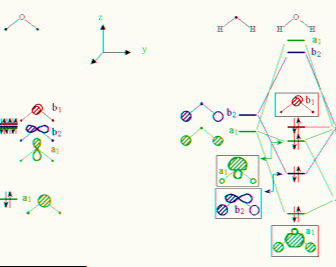
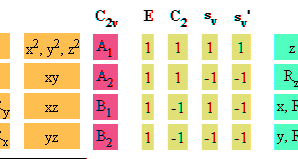
Character table for point group C2v, H2O, water molecule
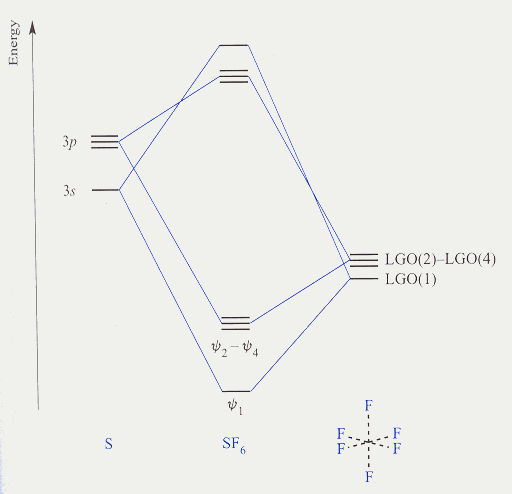
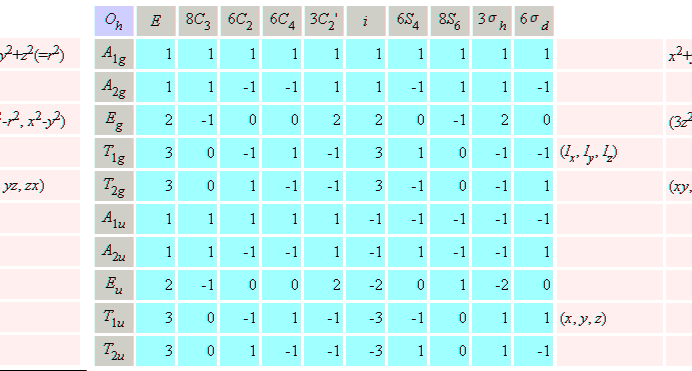
Character table for point group Oh, SF6,
10)
a) Using character table for point group C2v, H2O,
water molecule identify symmetry representation of s, px, py
and px atomic orbitals on oxygen atom of water molecule.
b) Using character table for point group Oh, SF6,
s, px, py px, dz2, dx2-y2,
dxy, and dxz, dyz atomic orbitals on sulfur
atom of SF6 molecule.

11) Comment on the symmetries of the molecular orbital diagrams of H2O and SF6