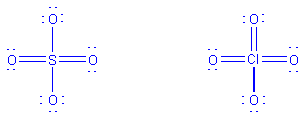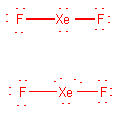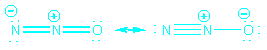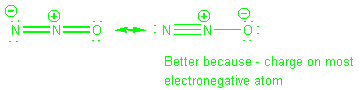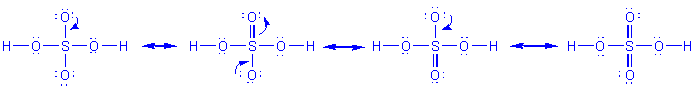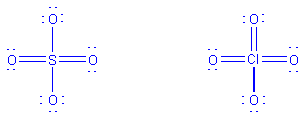Lewis Structures
For a thorough review of this material, see Chapter 3 of
Concepts
of Chemistry.
Basic Method
-
Arrange the atoms in the correct layout. This is either given
or can be guessed. For a molecule of formula AXn, A is usually
the central atom with the X atoms all attached to it.
-
Determine the number of electrons available from the group
numbers of the elements.
-
Draw an initial structure by
-
Putting single bonds between neighboring atoms;
-
Putting lone pairs on atoms to give each an octet (except
H) until the number of electrons counted above has been place in the structure
or until every atom has an octet.
-
Alter the initial structure if necessary
-
If the initial structure shows every atom with an octet,
the structure is correct
-
If one or more atoms in the initial structure have fewer
than 8 electrons, use lone pairs to form multiple (double, triple) bonds
to these atoms until every atom has 8 (except H)
-
If every atom has an octet but not all available electrons
have been used in the initial structure, add all remaining electrons to
the central atom. In these cases, the central atom will be from period
> 3.
Examples:
SO2
-
atom layout O-S-O
-
Count electrons: 6(S) + 2(6)(O) = 18
-
Draw initial structure:

-
Alter initial structure if necessary
As drawn, the initial structure shows sulfur with only
6 electrons. Use a lone pair from the oxygen atom on the right to form
a double bond to sulfur. This gives sulfur 8 electrons without changing
the number of electrons on the oxygen atom or the total number of electrons
in the structure (which must be 18). Note that we could just as well have
used a lone pair from the left oxygen.
SO42-
-
atom layout
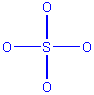
-
Count electrons: 6(S) + 4(6)(oxygen) + 2 (double negative
charge) = 32
-
Draw initial structure
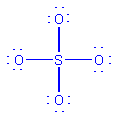
-
Alter initial structure if necessary. The initial structure
is fine as drawn, because it shows the correct total number of electrons,
and every atom has an octet.
XeF2
-
atom layout F-Xe-F
-
Count electrons: 8(Xe) + 2(7)(F) = 22
-
Draw initial structure (see below)
-
Alter initial structure if necessary.
As drawn, the initial structure shows every atom with
an octet, but with only 20 electrons. The remaining 2 electrons are placed
on Xe:
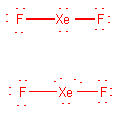
Formal charge (FC)
Guidelines
-
Avoid large formal charges on atoms
-
Avoid having FC of same sign on adjacent atoms.
-
Avoid large separation of opposite charges.
-
FCs should be consistent with atom electronegativities.
Consider N2O, for which the basic method gives
the following 3 possible structures.
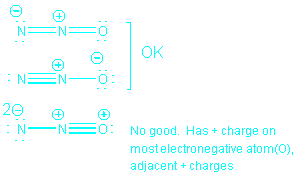
Resonance
When 2 or more reasonable Lewis structures can be drawn
for a molecule, the actual structure is a composite of the contributing
forms, called resonance forms. We indicate resonance using a double headed
arrow
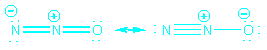
The actual structure is more stable than either of the
(hypothetical) contributing forms.
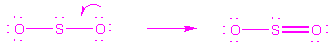
When 2 resonance forms are equivalent, they contribute
equally to the true structure. For example

When 2 forms are non-equivalent, they contribute in proportion
to their acceptability.
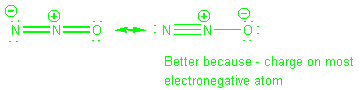
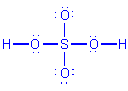
Procedure for developing resonance forms, applied to H2SO4:
-
Decide on an arrangement for the atoms. Once arranged,
the atoms may not be moved. For sulfuric acid, the arrangement is

-
Determine an acceptable Lewis structure, usually the one
resulting directly from the procedure presented above. For sulfuric acid,
this is
-
Move electrons only to achieve all other acceptable Lewis
structures. For sulfuric acid, there are 4:
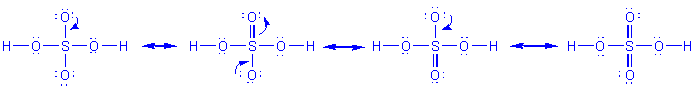
Is the following structure a resonance form for sulfuric
acid?
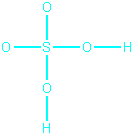
No, because a hydrogen atom has been moved. This structure
is the same as the structure in step 2 above.
Several resonance forms for the pyrimidine base, cytosine,
are shown below:
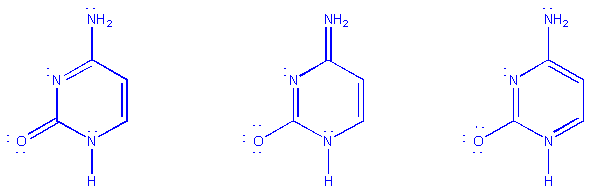
Note that the atoms are kept in fixed locations; only
electrons are moved.
The following structure is NOT a resonance form, because
a H atom has been moved:
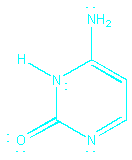
This is an isomer of cytosine. Isomers are different
arrangements of
atoms; resonance forms are different arrangements
of
electrons.
Refinements
When the central atom of a species is from period > 3,
it can exceed the octet. The best structure for such a species is usually
considered to be the one in which sufficient double bonds are drawn to
the central atom from the terminal atoms to give the central atom a formal
charge of zero. The following structures would thus be drawn for SO42-
and ClO4-: