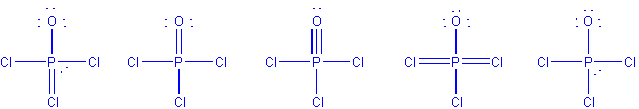
1. Which choice is an acceptable Lewis structure for POCl3
? Lone pairs on chlorines not shown.

Second from left
2. The diagram that best represents the molecular stereochemistry
of POCl3 is
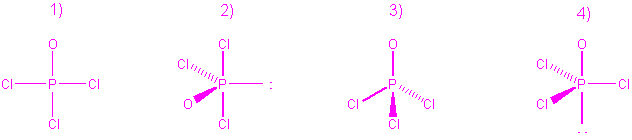
Number 3
3. Which compound is expected to be most ionic?
1) KCl 2) MgCl2 3) AlCl3 4) TiCl4 5) VCl5
4. Which cation produces the most acidic solution when its chloride is dissolved in water?
1) Na+ 2) Ti4+ 3) Al3+ 4) Mg2+ 5) Li+
5. Which diagram best represents a portion of a crystal
of NaCl? Explain your thinking in making your choice.

Third from left because has neighbors of cations are all anions and vice versa.
Questions 6-10 concern the following redox half reactions and their corresponding standard reduction potentials
1) Fe3+ + e ® Fe2+ e 0 = 0.77 V
2) Cu2+ + 2e ® Cu(s) e 0 = 0.34 V
3) Zn2+ + 2e ® Zn(s) e 0 = -0.76 V
4) 2H2O + 2e ® H2(g) + 2OH- e 0 = -0.83 V
5) Ni2+ + 2e ® Ni(s) e 0 = -0.23 V
6. The half reaction that is most spontaneous as written under standard conditions is 1).
7. The strongest oxidant (oxidizing agent) is in reaction 1).
8. The strongest reductant (reducing agent) is in reaction 4).
9. The half reaction whose reduction potential depends on pH is 4).
10. The reaction having the most positive standard free energy change is 4).
11. Ka values for H2CO3 are 4.3 x 10-7 and 4.4 x 10-11. Which species will predominate in an aqueous solution having pH = 8.4?
1) H2CO3 2) HCO3- 3) CO32- 4) H3O+ 5) OH-
12. Which substance is expected to have the lowest boiling point?
1) MgF2 2) PF3 3) F2 4) NaF 5) SiF4
13. Which fundamental property of the atom is primarily responsible for the fact that atoms become smaller from left to right across a period of the Periodic Table?
1) First ionization energy 2) First electron affinity 3) Enthalpy of atomization 4) Effective nuclear charge 5) Principal quantum number of the valence shell
14. Which substance is unlikely to function as a Lewis base?
1) H2O 2) CH4 3) NH3 4) SO2 5) (CH3CH2)2O (diethyl ether)
15. The stereochemistry of SF4 is best described as
1) tetrahedral 2) square planar 3) trigonal bipyramidal 4) trigonal planar 5) distorted tetrahedral
16. Which structure for P4S3 is
reasonable in terms of expected bonding patterns for sulfur and phosphorus?
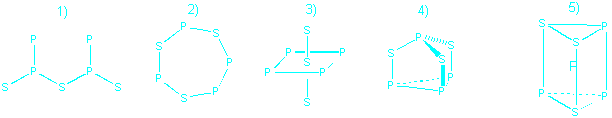
Number 4 because it has P making 3 bonds and S making 2.
17. Which hydrogen compound produces a basic solution when dissolved in water?
1) ClH 2) OH2 3) SO2(OH)2 4) CaH2 5) CH4
18. If one wanted to oxidize Cu(s) (copper metal) in aqueous medium, the best choice of oxidizing agent might be
1) O2(g) 2) H2SO4(aq) 3) S(s) 4) Fe(s) 5) HNO3(aq)
19. How many reasonable resonance forms can be drawn for HClO4?
1) one 2) two 3) four 4) seven 5) eight
20. Which Lewis acid is expected to have the greatest affinity for the Lewis base, S2-?
1) Na+ 2) Pb2+ 3) Pb4+ 4) Fe2+ 5) Fe3+
21. The LUMO of HCl is
1) A p antibonding orbital composed of Cl(3px) and H(1s)
2) A p bonding orbital composed of Cl(3py) and H(1s)
3) A s antibonding orbital composed of Cl(3pz) and H(1s)
4) A s bonding orbital composed of Cl(3s) and H(1s)
5) A s antibonding orbital composed of Cl(3s) and H(1s)
22. Silicate anions are extremely common in nature, being found in many naturally occuring minerals. The structures of all silicates are based on
1) Trigonal planar SiO3 units 2) Tetrahedral SiO4 units 3) Alternating SiO3 and SiO4 units 4) Linear units in which Si and O atoms alternate 5) Complex 3-dimensional arrangements consisting of SiO4 tetrahedra and SiO6 octahedra
23. Hybrid orbitals used by phosphorus in PCl5 are best described as
1) sp2 2) sp3 3) sp4 4) sp3d 5) sp3d2
24. The reaction PF5 +4H2O ® 5HF + H3PO4 is best described as
1) Lewis adduct formation 2) Lewis adduct dissociation 3) Diels-Alder addition 4) redox 5) double displacement
25. Which molecule would be predicted by VSEPR to have square planar stereochemistry?
1) SF4 2) CH4 3) PF4+ 4) BF4- 5) XeF4