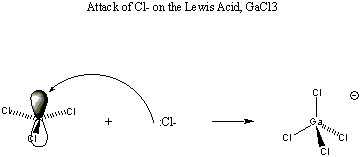
Definitions of Lewis Acid-Base Theory. Lewis acid/base theory (sometimes called donor-acceptor theory) is a broad, widely applicable approach to the classification of chemical substances and the analysis of chemical reactions. According to this theory, a base is defined as an electron pair donor, and an acid as an electron pair acceptor. Donation of an electron pair from base to acid results in the joining of the acid and base via a covalent bond. The bonded acid-base species is called an adduct (short for addition product), a coordination compound, or a complex. Lewis theory puts the emphasis on the donation and acceptance of electrons; this is appropriate, because it is the give and take of electrons (+ seeks – and avoids +) that drives all of chemistry. As we will see, there is often a change in stereochemistry at the acceptor atom when the adduct forms.
Recognition of Acids and Bases. Lewis Acids. Generally speaking, Lewis acids are not as easily recognized as are the bases, but there are certain "hallmarks" of Lewis acids to be alert for. In general, a species that contains a fully or partially positive atom with an empty or underused valence orbital is very likely to function as a Lewis acid--an electron pair acceptor. According to this idea, species such as H+, (CH3)3C+ (a carbocation), and Fe3+ are expected to function as Lewis acids, accepting one or more electron pairs into empty valence orbitals. The positive charge of the acid serves to attract the lone electron pair of the base into the vicinity of the acid so that orbital overlap and bonding may take place. The first two of these acids have a single open valence orbital (1s in the case of H+, 2pz in the case of (CH3)3C+), so are capable of interacting with only one Lewis base. Fe3+, on the other hand, readily accepts up to 6 lone pairs into empty 4s, 4p, and 4d valence orbitals. For example, dissolution of a salt of Fe3+ in water results in the following process:
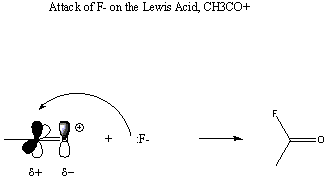
Less obvious examples of Lewis acids are CO2, SnCl2, and SO3, None of these species is positively charged. However, in all cases the central atom is attached to electronegative terminal atoms and therefore carries a partial positive charge. In addition, in each case the central atom is involved in at least one pi bond with the terminal atoms. Because pi bonds are substantially weaker than sigma bonds, we regard the carbon p orbitals used in these bonds to be underused. All of these species are subject to attack by a Lewis base at the pi-bonding p orbital of the central atom. This results in disruption of the pi bond and its replacement by a stronger sigma bond between the attacking base and the central atom of the acid, as shown below:
Finally, species such as BF3, AlCl3, and PF5 are Lewis acids because in all cases the central atom bears a partial positive charge, and there is at least one unused valence orbital on the central atom. In the first 2 species, the unused orbital is the valence pz orbital of B or Al. In PF5, there are 4 unused valence 3d orbitals that may be used to accept electron pairs.
Lewis Bases. Lewis bases (ligands) are quite easy to recognize; they are species that contain atoms with lone pairs. Such atoms are called donor atoms. Thus H2O (donor atom, O), NH3 (donor atom, N), Cl-, and CH3CH2OH (donor atoms, O) are immediately recognized as Lewis bases. Because the number of Lewis bases is almost unlimited, it is useful to categorize them based on the number of donatable nonbonding electron pairs that they contain.
1) Monodentate (1-toothed) bases (ligands). These are ligands that donate one electron pair to one acid ion or molecule. Examples of monodentate ligands include anions such as OH-, O2-, NH2-, S2-, HS-, CH3-, H-, X-; neutral molecules such as H2O, NH3, CO, CH3CH2OH, (CH3)2C=O, (CH3CH2)2O (and many more); and species with relatively high energy p electrons, such as olefins.
2) Polydentate (many-toothed) ligands. A polydentate ligand, sometimes called a chelating ligand, has 2 or more donor atoms spaced so that they can attach to the same Lewis acid. Normally the donor atoms must be "spaced" by 2 or 3 intervening atoms. Polydentate ligands are subdivided more precisely as follows.
a) Bidentate (2-toothed) ligands. The prototype for bidentate ligands is ethylenediamine, H2NCH2CH2NH2. This molecule donates both N atoms to the same Lewis acid to give a 5-membered ring, as shown. A second example is the dianion of aspartic acid, H2NCH(CH2COO-)COO-, which can conceivable be bidentate in a couple of ways. Draw a careful structure of the molecule, identify the potential donor atoms, and determine the ways in which aspartate can be bidentate. A third example of a bidentate ligand is 1,10-phenanthroline. Because many metal ions use 6 (of 9 possible) coordination positions, they can accommodate up to 3 bidentate ligands. Thus adducts such as Ni(en)32+ and Ni(phen)32+ are common.
b) Tridentate ligands. Examples of potentially tridentate ligands include diethylenetriamine (abbreviated dien) and 2,6-pyridinedicarboxylate. The structures are shown.
c) Tetradentate ligands have four appropriately spaced donor atoms. Such ligands can be linear, branched, or cyclic. A linear tetradentate ligand is one in which all 4 donor atoms occur in a single chain of ligand atoms. The prototype for the linear tetradentate ligand is triethylenetetramine, H2N-CH2-CH2-NH-CH2-CH2-NH-CH2-CH2-NH2. A second and interesting example is the anion, bis(salicylaldehyde)ethylenediimine (abbreviated salen). Coordination of the salen anion to a transition metal ion results in adducts with interesting physical and chemical properties. Tetradentate ligands can also have a branched structure, meaning that donor atoms are located in 2 or more atom chains in the molecule. An example is tris-(2-aminoethyl)amine, which has the tripod-like shape shown. This ligand tends to retain its tripod-like arrangement even when bound to a metal ion. It can therefore be used to force a specific coordination geometry on the metal ion. Finally, many tetradentate ligands are (macro)cyclic in structure. A macrocyclic ligand is a chelate ligand with the donor atoms in a ring. The most famous example is the porphyrin, a naturally occurring macrocyclic ligand of great importance. In addition, a large variety of synthetic macrocyclic ligands has been designed and studied as models for the porphyrin ligand. Interesting chemistry has been discovered with Lewis adducts of these macrocycles, some of which are shown.
d) Hexadentate ligands offer six donor atoms to an acid center. The best known of these is ethylenediaminetetraacetate (abbrev. EDTA). This ligand is used as a sequestering (gathering) agent in water treatment, food preservation, analysis, and heavy metal poisoning. It functions by tying up metal ions that might otherwise precipitate with CO32- (water treatment), catalyze undesirable reactions (food preservation), or interfere with enzyme and protein function (heavy metal poisoning).
It is very generally true that for a specified type of donor atom in the ligand (for example, N), stabilities of adducts with a particular metal ion acid tend to increase as follows:
Exercise. Classify as Lewis acids and/or bases: pyridine; DMSO, COCl2, benzene, SCN-.
Interpretation of Reactions in Terms of Lewis Theory. To illustrate the applicability of Lewis acid/base theory, we will analyze several simple and familiar reactions in terms of Lewis ideas.
Example: C6H6 + CH3Cl ® C6H5CH3 + HCl, carried out in the presence of AlCl3 as a catalyst. The generally accepted mechanism for this process involves several steps, each step involving a Lewis acid-base interaction.
Step 2: C6H6 + CH3+ ® C5H5C(H)(CH3)+, in which the methyl carbocation has formed a sigma bond with one carbon atom of the benzene ring by accepting one of the 3 pairs of pi electrons of the ring in a Lewis acid-base reaction.
Step 3: C5H5C(H)(CH3)+ ® C6H4(CH3) + H+. In this process, the adduct of H+ with toluene dissociates.
Step 4: H+ + AlCl4- ® HCl + AlCl3. H+ strips the Lewis base, Cl-, from the adduct AlCl4- to form a new adduct, HCl, and regenerate the catalyst, AlCl3.
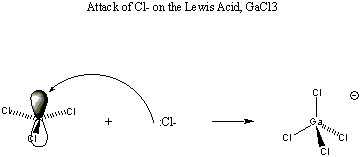
Fundamentals. The basic premise of Hard/Soft Acid/Base Theory is very simple: Hard acids prefer hard bases; soft acids prefer soft bases. We must now define these terms.
Hard acids (in context, HA) are characterized by (s,f blocks, left side of d block in higher OS's)
Hard bases (in context, HB) are characterized by
Soft acids (in context, SA) are characterized by an acceptor atom of
Soft bases (in context, SB) are characterized by donor atom of
In addition to the fundamental "hard" and "soft" categories, two additional categories are useful. Borderline acids (in context, BA) are intermediate between hard and soft acids. Thus they tend to have lower charge and somewhat larger size than hard acids, and higher charge and somewhat smaller size than soft acids. The 2+ ions of the d block, such as Fe2+, Cu2+, Ni2+, and Zn2+, are borderline acids. Borderline bases (in context, BB) are intermediate between hard and soft bases. They tend to be larger and less electronegative than hard bases, smaller and more electronegative than soft bases. Bases in which the donor atom is N or Cl fall in this category. Thus NH3, Cl-, RCl, and pyridine are borderline bases.
Example: Classify each species as Lewis acid or base; as hard, soft, or borderline. Which of the Lewis bases would prefer to form adducts with each of the acids?
I-: This is a large anion with low electronegativity and low charge; it is expected to be a soft base.
CH3-: This is an anion, so is probably a base. The donor atom has low electronegativity and relatively low charge. Even though the donor atom is fairly small, this behaves as a soft base.
CO32-: This is an anion with oxygen atoms as potential donors. It is a hard base.
Cu+: This is a transition metal cation with a low charge. It is expected to be a soft acid.
Cl-: This is an anion with a chlorine donor atom. It should be a borderline base.
Se(CH3)2F-: This is an anion with two potential donor atoms: F, which is a hard donor; and Se, with two electron pairs, which is expected to be a soft base. This species is definitely a base, but can be soft or hard depending on circumstances.
Because hard acids and bases tend to be highly charged and nonpolarizable, the interaction between them is largely ionic. Their small sizes allow the acid and base to get close enough together so that the ionic interaction is quite strong (remember Coulomb's Law: force of attraction increases as the attracting species get closer together). A good image to keep in mind when thinking of a hard acid/hard base adduct is the juxtaposition of a golf ball (the "acid") with a baseball (the base). Both of these items are small, hard, and undistortable (try pushing the golf ball and baseball together--what happens?). In contrast, soft acids and soft bases have covalent interactions because their electron clouds are polarizable. A good image for soft-soft interactions is the juxtaposition of a large nerf ball (the base) with a smaller nerf ball (the acid). Here the electron clouds are squishy, and can be easily distored during the interaction (try pushing the 2 nerf balls together--what happens?).
Refinements to HSAB Theory: Factors Influencing Hardness/Softness. Before considering some broad applications of the HSAB ideas, we will add some refinements to the major principle that "hard acids prefer hard bases; soft acids prefer soft bases".
1) There are degrees of hardness and softness. Hardness/softness of acids is affected by several factors. First, the nature of the acceptor atom. Thus Au+ > Hg2+ > Pb2+ in softness. Hardness increases with distance from gold. Second, the charge: hardness increases with increasing charge (oxidation state). For example, Tl3+ is harder than Tl+. Third, the attached groups: Attaching soft substituents (bases) to an acceptor atom increases the softness of the acceptor atom; attaching hard bases increases the hardness of the acceptor atom. Thus, for example, AlF3 is harder than Al(CH3)3
Exercise: Order the following acids according to softness:
Exercise: Order the following bases according to softness:
2) Stronger acids prefer stronger bases. This often complicates application of the HSAB guidelines. Strengths of acids and bases can take precedence over hard/soft rules, particularly in cases where only one soft species is present. For example, Bronsted-Lowry acid-base reactions always involve a hard acid (H+) and mostly hard bases (e.g., H2O, OH-) so are not predictable using HSAB. The following reaction illustrates the point.
Example: HCl (HA-BB) + HOH:OH2 (HA-HB) ® H3O+ (HA-HB) + HOH-Cl- (HA-BB)
where HA = hard acid, etc. Although HSAB ideas are not applicable here, the direction of reaction can easily be predicted using relative acid and base strengths.
Applicability of HSAB Theory. The HSAB principle applies to exchange (double displacement) reactions, represented generally as follows:
Example: Ni(H2O)62+ (BA-HB) + H3N-HOH (HA-BB) ® Ni(NH3)62+ (BA-BB) + HOH-OH2 (HA-HB)
With solvation explicitly represented, this reaction can be seen to favor products. HOWEVER, the principal is not applicable when there is only one soft species (acid or base) involved. In terms of HSAB ideas, we can say nothing about whether reactants or products are favored in the following reaction.
1) Predicting favorable equilibria.
Example: In the following situation, which product will form, LiI or LiF?
Example: In the following situation, which product will form?
Example: In the following situation, what if anything can we predict will happen?
Exercise: Is the HSAB principle applicable to the following situation?
| Acids | Bases | Adducts |
| Hg2+ (SA) | Cl- (BB) | HgI2 |
| Na+ (HA) | I- (SB) | Na(H2O)6+ |
| H2O (HA) | H2O (HB) | (H2O)n
Cl -
HOH-OH2 |
| Acids | Bases | Adducts |
| Ag+ (SA) | NO3- (HB) | AgCl |
| K+ (HA) | Cl- (BB) | K(H2O)6+ |
| H2O (HA) | H2O (HB) | (H2O)n
NO3-
HOH-OH2 |
2) Geochemistry of the elements. In nature, hard acids are found associated with hard bases, and soft acids are found associated with soft bases. Thus hard acids tend to occur as oxides, silicates, carbonates, and fluorides, whereas soft acids occur as sulfides, selenides, and tellurides, or as free elements (in which case they can be viewed as cations associated with electrons, which are very soft bases). Borderline acids often occur as sulfides. Metals found in nature in combination with the group 16 elements S, Se, and Te are sometimes called chalcophiles.
Exercise: Predict the type of ore in which each metal ion is likely to be found: Fe3+, Fe2+, Hg2+, Zn2+, Na+, Ca2+, Si4+.
3) Toxicology, Medicinal Chemistry. Ions of many so-called heavy metals, such as Hg2+ and Pb2+, are highly toxic. Why? Heavy metal ions are soft acids, and therefore have high affinity for S2-, a soft base. S occurs in the side chains of two amino acids, methionine and cystine, and is important in maintaining tertiary structure of proteins and enzymes upon which life depends. Ingested heavy metal ion seeks out and coordinates with amino acid sulfur, disrupting protein structure and deactivating the protein. Eventual death is the usual result of prolonged exposure to heavy metal ions. As an illustration of the affinity of heavy metal ions for sulfur, we consider the Hg2+ cation. The solubility product for HgS in aqueous solution is about 10-50 M2, meaning that HgS has a solubility of 1 x 10-25 M in water. Think about this number. It means that in one liter of such a solution, there will be less than 1 Hg2+ ion! Based on this solubility, we can calculate that to dissolve 1.0 g HgS would require 4 x 1022L H2O. But there are only 1 x 1021 L of water on earth!!!
Exercise: Why are HCN, CO, H2S, H2Se, and PH3 poisons?
4) Ligand selections in metalloproteins and enzymes. Ions of many of the 3d transition metals are essential for life in trace amounts. A number of proteins and enzymes incorporate these metal ions specifically into their structures, forming adducts with the metal ion using donor atoms on the side chains of their amino acids. Side chains containing oxygen, nitrogen, and sulfur donors are usually involved in adduct formation. It is interesting to apply HSAB ideas to attempt to understand why certain amino acids are frequently found with their side chain donors bound to particular metal ions. As a particular example, consider the Fe2+ ion, perhaps the most-used metal ion in life processes. This ion is found in species such as myoglobin and hemoglobin, in which is function is to accept an electron pair from an O2 molecule to form an adduct, carry the O2 to the muscle tissues of the body, and release the O2 unaltered where it is needed. It is found in electron transfer proteins such as cytochrome c, where its function is to mediate the movement of an electron between other proteins. It is found in the iron-sulfur proteins, where its function is again redox related. In all of these varied proteins, with their different functions, the Fe2+ ion is invariably associated with N and S donor atoms. This is sensible in terms of HSAB theory, because Fe2+ is a borderline acid. Its association with the borderline donor, N, and the soft donor, S, is therefore understandable. Similarly, the Zn2+ and Cu2+ ions are attached to proteins and enzymes via N and O donors. Fe3+, on the other hand, is a hard acid and is invariably found bound by O donors.
Exercise: What donor atoms might be appropriate for binding Cu+ in an enzyme or protein? Na+? K+? Ca2+?
Interestingly, Cu2+ is found in the so-called blue copper proteins coordinated by N and S donors, and it is natural to wonder why a soft donor atom is involved? The purpose of the blue copper proteins is electron transfer, requiring that the copper ion cycle back and forth between the 1+ and 2+ oxidation states. The coordination sphere of the ion is thus optimally designed to accommodate both oxidation states: S donors are there to stabilize Cu+, and N donors are there to stabilize Cu2+.
5) Reduction Potentials. The electron has been termed the "ultimate soft base". Viewing the electron in these terms, standard reduction potential can be understood in terms of HSAB theory. Several standard reduction potentials are given below.
Ex: Arrange the following half reactions in order of decreasing standard reduction potential. "Decrease" means "to become less positive". After you have predicted an order, look up the SRPs to see if you were correct!