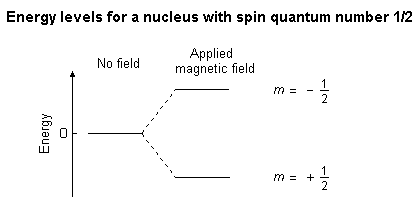

When the nucleus is in a magnetic field, the initial populations of the
energy levels are determined by thermodynamics, as described by the Boltzmann
distribution. This is very important, and it means that the lower energy
level will contain slightly more nuclei than the higher level. It is
possible to excite these nuclei into the higher level with electromagnetic
radiation. The frequency of radiation needed is determined by the difference in
energy between the energy levels.
The constant, g, is called the magnetogyric
ratioand is a fundamental nuclear constant which has a different value for
every nucleus. h is Plancks constant.
The energy of a particular
energy level is given by;
Where B is the strength of the magnetic field at the nucleus.
The difference in energy between levels (the transition energy) can be found from
This means that if the magnetic field, B, is increased, so is DE. It also means that if a nucleus has a relatively large magnetogyric ratio, then DE is correspondingly large.
If you had trouble understanding this section, try reading the next bit (The
absorption of radiation by a nucleus in a magnetic field) and then come back.
Imagine a nucleus (of spin 1/2) in a magnetic field. This nucleus is in the lower energy level (i.e. its magnetic moment does not oppose the applied field). The nucleus is spinning on its axis. In the presence of a magnetic field, this axis of rotation will precess around the magnetic field;
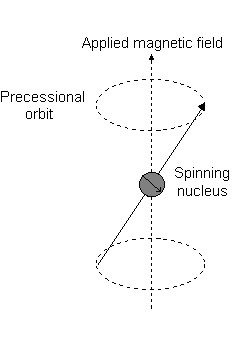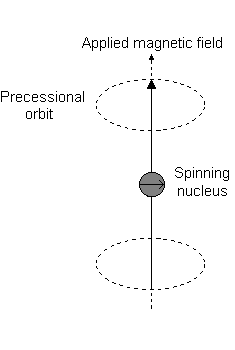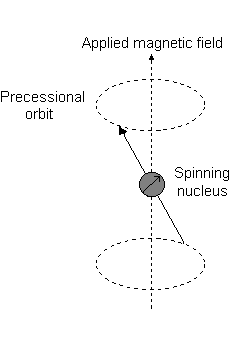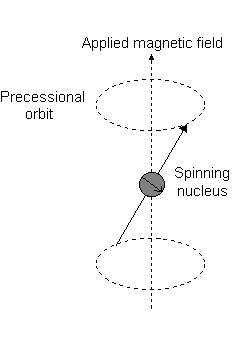
The frequency of precession is termed the Larmor frequency, which is identical to the transition frequency.
The potential energy of the precessing nucleus is given by;
E = - m B cos qwhere q is the angle between the direction of the applied field and the axis of nuclear rotation.
If energy is absorbed by the nucleus, then the angle of precession, q, will change. For a nucleus of spin 1/2, absorption of radiation "flips" the magnetic moment so that it opposes the applied field (the higher energy state).
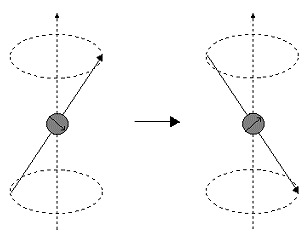
It is important to realise that only a small proportion of "target" nuclei
are in the lower energy state (and can absorb radiation). There is the
possibility that by exciting these nuclei, the populations of the higher and
lower energy levels will become equal. If this occurs, then there will be
no further absorption of radiation. The spin system is saturated.
The possibility of saturation means that we must be aware of the relaxation
processes which return nuclei to the lower energy state.
Ideally, the NMR spectroscopist would like relaxation rates to be fast - but not too fast. If the relaxation rate is fast, then saturation is reduced. If the relaxation rate is too fast, line-broadening in the resultant NMR spectrum is observed.
There are two major relaxation processes;
The relaxation time, T1 (the average lifetime of nuclei in the higher energy state) is dependant on the magnetogyric ratio of the nucleus and the mobility of the lattice. As mobility increases, the vibrational and rotational frequencies increase, making it more likely for a component of the lattice field to be able to interact with excited nuclei. However, at extremely high mobilities, the probability of a component of the lattice field being able to interact with excited nuclei decreases.
Spin - spin relaxation
Spin - spin relaxation describes the
interaction between neighbouring nuclei with identical precessional frequencies
but differing magnetic quantum states. In this situation, the nuclei can
exchange quantum states; a nucleus in the lower energy level will be excited,
while the excited nucleus relaxes to the lower energy state. There is no
net change in the populations of the energy states, but the average
lifetime of a nucleus in the excited state will decrease. This can result in
line-broadening.
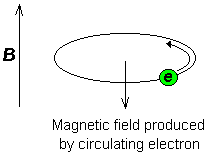
Consider the s-electrons in a molecule. They have spherical symmetry and circulate in the applied field, producing a magnetic field which opposes the applied field. This means that the applied field strength must be increased for the nucleus to absorb at its transition frequency. This upfield shift is also termed diamagnetic shift.
Electrons in p-orbitals have no spherical symmetry. They produce comparatively large magnetic fields at the nucleus, which give a low field shift. This "deshielding" is termed paramagnetic shift.
In proton (1H) NMR, p-orbitals play no part (there aren't any!), which is why only a small range of chemical shift (10 ppm) is observed. We can easily see the effect of s-electrons on the chemical shift by looking at substituted methanes, CH3X. As X becomes increasingly electronegative, so the electron density around the protons decreases, and they resonate at lower field strengths (increasing dH values).
Chemical shift is defined as nuclear shielding / applied magnetic field. Chemical shift is a function of the nucleus and its environment. It is measured relative to a reference compound. For 1H NMR, the reference is usually tetramethylsilane, Si (CH3)4.
The 1H NMR spectrum of ethanol (below) shows the methyl peak has been split into three peaks (a triplet) and the methylene peak has been split into four peaks (a quartet). This occurs because there is a small interaction (coupling) between the two groups of protons. The spacings between the peaks of the methyl triplet are equal to the spacings between the peaks of the methylene quartet. This spacing is measured in Hertz and is called the coupling constant, J.
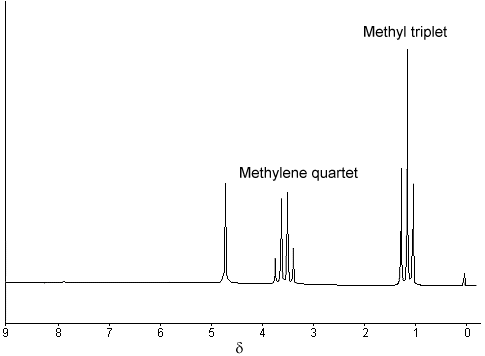
To see why the methyl peak is split into a triplet, let's look at the methylene protons. There are two of them, and each can have one of two possible orientations (aligned with or opposed against the applied field). This gives a total of four possible states;
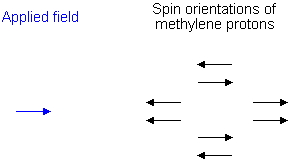
In the first possible combination, spins are paired and opposed to the field. This has the effect of reducing the field experienced by the methyl protons; therefore a slightly higher field is needed to bring them to resonance, resulting in an upfield shift. Neither combination of spins opposed to each other has an effect on the methyl peak. The spins paired in the direction of the field produce a downfield shift. Hence, the methyl peak is split into three, with the ratio of areas 1:2:1.
Similarly, the effect of the methyl protons on the methylene protons is such that there are eight possible spin combinations for the three methyl protons;
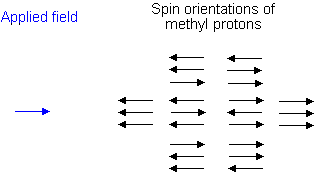
Out of these eight groups, there are two groups of three magnetically equivalent combinations. The methylene peak is split into a quartet. The areas of the peaks in the quartet have the ration 1:3:3:1.
In a first-order spectrum (where the chemical shift between interacting groups is much larger than their coupling constant), interpretation of splitting patterns is quite straightforward;