Infra-Red (IR) Spectroscopy
An IR spectrum
is produced by absorption of energy (4000-400 cm-1) due to vibrations
of polar covalent bonds of inoganic or organic molecules. This provides an
important method for identifying the functional groups within an organic
molecule, the region 4000-1000 cm-1 is most useful for this. Below
1000 cm-1 the spectra tend to be more complex and hard to assign.
However, this fingerprint region can be used to confirm a structure by
direct comparison (known vs unknown).The most useful information you should be
able to read in an IR spectrum is what functional groups are present. Remember
that different functional groups can be "viewed" as combinations of bond types,
e.g. an ester is C=O and C-O.
Avoid the temptation to try to assign every
peak.
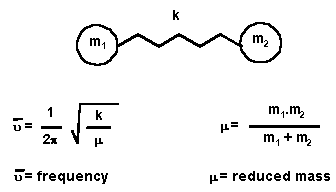 To help understand IR it is useful to use a Hooke's Law model, as shown
to the left. Consider a bond and the connected atoms to be a spring (force
constant k) and two masses (m1 and m2), then the equation indicates how the
frequency of the absorption should change for a stronger bond (larger k value)
and as the masses involved change.
To help understand IR it is useful to use a Hooke's Law model, as shown
to the left. Consider a bond and the connected atoms to be a spring (force
constant k) and two masses (m1 and m2), then the equation indicates how the
frequency of the absorption should change for a stronger bond (larger k value)
and as the masses involved change.
As examples of this look at the positions
of C-C (1000 cm-1), C=C (1600 cm-1) and C#C (2200
cm-1), and the positions of C-H (3000 cm-1) and C-C (1000
cm-1).
The absorptions you should learn to recognise, in order of
importance are:
|
Bond |
Base Value |
Strength / Shape |
Comments |
|
1 |
C=O |
1715 |
s, "finger" |
Exact position depends on type of carbonyl |
|
2 |
O-H |
3600 |
s, brd |
Broad due to H bonding |
|
3 |
N-H |
3500 |
m |
Can tell primary from secondary |
|
4 |
C-O |
1100 |
s |
Also check for OH and C=O |
|
5 |
C=C |
1650 |
w alkene
m-s aromatic |
Alkene w due to low polarity
Aromatic usually in
pairs |
|
6 |
C#C |
2150 |
w, sharp |
Most obvious in terminal alkynes |
|
7 |
C-H |
3000 |
s |
As hybridisation of C changes sp3-sp2-sp, the frequency
increases |
|
8 |
C#N |
2250 |
m, sharp |
Characteristic since little else around
it |
(# means triple bond here)
If
you know these, then you can identify most of the functional groups we are
interested in. Note that it is rarely useful to look for C-C since most organic
molecules will have them.
Be aware that the exact substitution pattern of a
particular bond causes shifts in the position of the absorption and therefore
ranges of values are given in the tables. You will normally be given copies of
the tables in exams, but you will save yourself lots of time if you know
approximately where the various bonds absorb.
It is possible to rationalise
the shifts of absorbances based on electronic effects due to proximal groups,
conjugation and / or ring strain.
In general, when you are trying to work
out what a molecule is, you will not just have the IR spectrum, but you will
have other information as well, such as the formula or most likely the NMR.
Always cross check between these sets of information, e.g. If the
formula has only one O, then you cannot have an ester (CO2R) or an
acid (CO2H)! or you should be able to distinguish an aldehyde and a
ketone by checking the proton NMR (9-10 ppm) !
 To help understand IR it is useful to use a Hooke's Law model, as shown
to the left. Consider a bond and the connected atoms to be a spring (force
constant k) and two masses (m1 and m2), then the equation indicates how the
frequency of the absorption should change for a stronger bond (larger k value)
and as the masses involved change.
To help understand IR it is useful to use a Hooke's Law model, as shown
to the left. Consider a bond and the connected atoms to be a spring (force
constant k) and two masses (m1 and m2), then the equation indicates how the
frequency of the absorption should change for a stronger bond (larger k value)
and as the masses involved change.