

Chapter 6-12

Chemistry
120 Online LA Tech
Fig. 6.9 In solving chemical-equation-based problems, the only “transitions” allowed are those between quantities (boxes) connected by arrows.
Mole
conversion to grams and particles
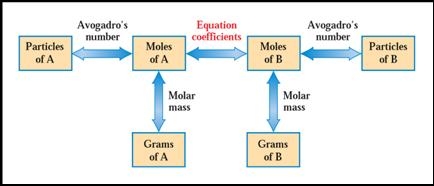
•Moles multiplied by Avogadro's number gives number of atoms and molecules( mole x N =
particles)
•Mole multiplied formula mass gives
grams. ( mole x F.M. = grams)
•Grams divided by formula mass gives
moles. ( grams ¸ F.M. = mole)