

Chapter 5-5

Chemistry
120 Online LA Tech
Covalent and
Coordinate covalent Bond
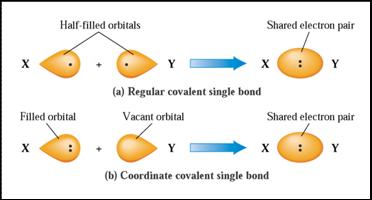
Fig. 5.3 (a) A
“regular” covalent single bond is the result of overlap of two half-filled orbitals. (b) A coordinate covalent
single bond is the
result of overlap of a filled and a vacant orbital.