1-7

Chemistry
121, Winter 2008, LA Tech
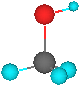
Alcohols - Structure
The
functional group of an alcohol is an -OH group bonded to an sp3 hybridized carbon
•bond angles about the hydroxyl oxygen atom are approximately 109.5°
Oxygen
is also sp3 hybridized
•two sp3 hybrid orbitals form sigma bonds to carbon and hydrogen
•the remaining two sp3 hybrid orbitals each contain an unshared pair of electrons