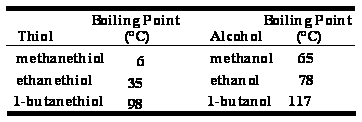1-59

Chemistry
121, Winter 2008, LA Tech
Thiols - Physical Properties
The
difference in electronegativity between S and H is 2.5 - 2.1 =
0.4
Because
of their low polarity, thiols
•show little association by hydrogen
bonding
•have lower boiling points and are less soluble
in water than
alcohols of comparable MW