1-8

Chemistry
121, Winter 2008, LA Tech
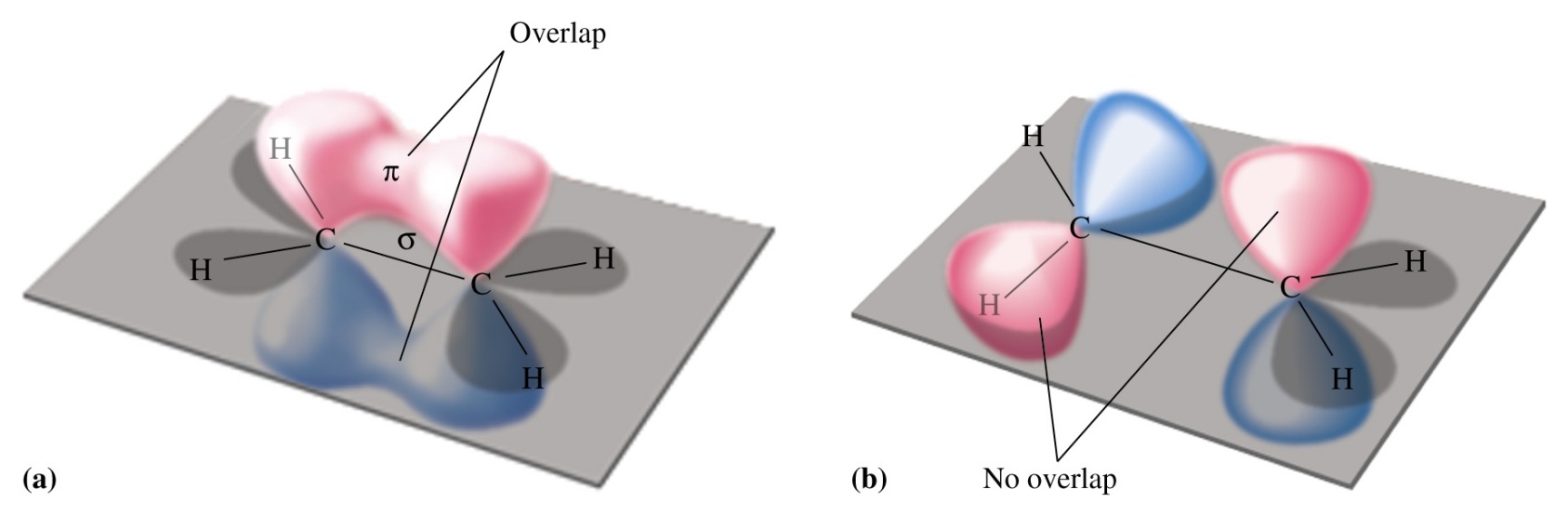
Planar Structure of Alkenes
According to the orbital overlap model, a double bond
consists of
•a s bond formed by overlap of sp2 hybrid orbitals
•a p bond formed by overlap of parallel 2p orbital
Rotating by 90°breaks the pi
bond