

1-47

Chemistry
121, Winter 2008, LA Tech
Conformations of cyclopentane
•In planar
cyclopentane, there are 10 fully eclipsed C-H bonds, which
create torsional strain of approximately 10 kcal/mol
•Puckering to an “envelope” conformation
relieves part of this strain
•In an envelope
conformation, eclipsed interactions are reduced but angle strain is
increased slightly (105°)
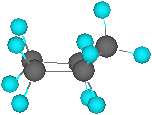
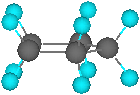
Envelope
conformation
Planar
conformation