

1-22

Chemistry
121, Winter 2008, LA Tech
•Primary (1°) carbon: a C
bonded to one other carbon
Secondary (2°) carbon : a
C bonded to two other carbons
Tertiary (3°) carbon : a
C bonded to three other carbons
Quaternary (4°) carbon : a C bonded to four other carbons
•
•
•
•
•Primary (1°) hydrogen: a H
bonded to primary (1°) carbon
Secondary (2°) hydrogen : a
H bonded to secondary (2°) carbon
Tertiary (3°) hydrogen : a
H bonded to tertiary (3°) carbon
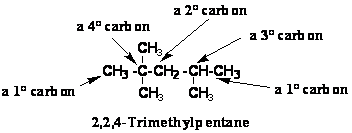
Types of carbon and hydrogen atoms