

Chapter 10-‹#›

Chemistry 120 Online LA Tech
Fig. 10.14 (a) The buffered and unbuffered solutions have the same pH level.
Buffers solutions compared to unbuffere
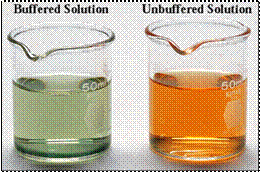
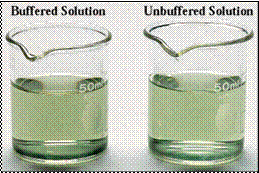
Fig. 10.14 (b) After adding 1mL of a 0.01 M HCl solution, the pH of the buffered solution has not perceptibly changed, but the unbuffered solution has become acidic.
Ken O’Donoghue © Houghton Mifflin
Company
Ken O’Donoghue © Houghton Mifflin
Company