1-37

Chemistry
121, Winter 2008, LA Tech
Acids
and Bases
There are many compounds when dissolved in water changes the Hydrogen ion (H+) (related to pH) concentration of to water to acid or basic sides
a)Binary acids: E.g. HF, HCl, HBr, HI, H2S
b)Oxyacid: E.g. HNO3,
H2SO4 , HClO4
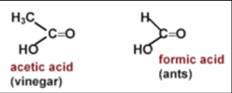
c)Organic acids: E.g.
d) Hydroxy bases: NaOH,
Ca(OH)2
e) Amine bases:
CH3NH2 (methylamine)
(CH3)2NH
(dimethylamine)