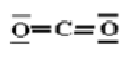|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
| Draw
Lewis structure of CO2 |
||||||||||
| i)
Valence electrons: 4 + 2 x 6
= 16 ( 8 pairs) |
||||||||||
| ii) Central atom C; O -- C -- O |
||||||||||
| iii) Give octet to carbon |
||||||||||
| -- |
||||||||||
| O -- C -- O |
||||||||||
| -- |
||||||||||
| Try to fill octet to O |
||||||||||
| iv)
Count electrons: |
||||||||||
| 4 bond pairs = 4
pairs |
||||||||||
| 4 lone pairs = 4 pairs |
||||||||||
| 8 electron
pairs |
||||||||||