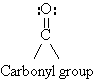
![]()
- >C=C< >C=O
- carbon is sp2 hybridized
- 120o bond angles
- planar about the double bond
Polar
d+
d-
+ -
>C==O « >C—O
- C is electrophilic
- O is nucleophilic
| >C=O
|
Similar to alkenes
- >C=C< >C=O - carbon is sp2 hybridized - 120o bond angles - planar about the double bond Polar
- C is electrophilic
|
Classification of Carbonyl Compounds by R- and Y-
All carbonyl compounds contain an acyl group , RCO- ,
bonded to another residue, -Y.
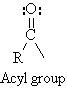 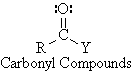 |
R- = alkyl, aryl alkenyl or alkynyl
Y- = C, H, O, N, S, halogen, or other atom Aldehyde: Y = H Ketone: Y = R group |
Classification of Carbonyl Compounds by Rxn Types
A. Y = non-leaving groups. (e.g. aldehydes and ketones)
B. Y = leaving groups. (e.g. -OR, -NR2,
-Cl)
Aldehydes: R-CHO Ar-CHO Ketones: R-COR' Ar-COR Ar-COAr'
Aldehyde Nomenclature
1. Identify the longest continuous chain of carbons with
the carbonyl carbon as part of the chain.
2. Assign priority (number the carbon chain) so that
the carbonyl (acyl) carbon is always #1. In nomenclature the carbonyl has
higher priority than the hydroxyl group.
3. Locate and identify alphabetically the branched groups
by prefixing the carbon number it is attached to. If more than one of the
same type of branched group is involved use the Greek prefixes di for 2,
tri for three, etc.
4. After identifying the name, number and location of
each branched group, use the alkane name corresponding to the number of
carbons in the continuous chain.
5. Drop the "e" and add the characteristic IUPAC ending
for all aldehydes, "al".
6. Alkenals involving Pi bonding will require that the
Pi bond is located but the ending will still be "al".
7. If the -CHO group is attached to a ring the suffix
-carbaldehyde is used.
|
(common name) |
(common name) |
| Methanal (formaldehyde) | 3-bromo-5-methylhexanal |
| Ethanal (acetaldehyde) | 2-methyl-4-hexenal |
| Propanal (propionaldehyde) | 3-hydroxybutanal (aldol) |
| Butanal (butryaldehyde) | Cyclohexanecarbaldehyde |
| Pentanal (valeraldehyde) | Benzenecarbaldehyde (benzaldehyde) |
| Propenal (acrolein) |
Ketone Nomenclature
1. Identify the longest continuous chain of carbons with
the carbonyl carbon as on of the carbons in the chain.
2. Number the carbons so that the carbonyl carbon has
the lowest possible number. If it makes no difference to the carbonyl carbon
then number so that the branched groups are attached to the lowest possible
carbon numbers.
3. Identify the branched groups by naming them in alphabetical
order and prefixing the carbon number they are attached to onto the name.
If more than one of the same group is present use the Greek prefixes (di
for 2 tri for 3, etc).
4. Use the alkane name corresponding to the number of
carbons in the chain and prefix the carbon number that the carbonyl carbon
has to the front of the alkane name.
5. Drop the "e" in the alkane name and add the characteristic
IUPAC ending for ketones which is "one".
6. When it is necessary to refer to the RCO- group as
a substituent, the term acyl is used and the name ending -yl is attached.
For example, CH3CO- is an acetyl group, -CHO is a formyl group,
and C6H5CO is a benzoyl group. If other functional
groups are present and the doubly bonded oxygen is considered a substituent,
the prefix oxo- is used.
| Propanone (acetone) | 2-Butanone (methy lethyl ketone) |
| 4,4-dimethyl-2-pentanone | Acetophenone (methyl phenyl ketone) |
| cyclopentanone | Benzophenone |
| 1-bromo-5-methyl-3-heptanone | Methyl-3-oxohexanoate
CH3CH2CH2COCH2CO2CH3 |
| 4-hydroxy-2-cylohexenone |
A. Aldehyde Synthesis
A1. Oxidation of primary alcohols by pyridinium chlorochromate
(PCC) in dichloromethane.
PCC
R-CH2-OH -----------> R-CHO
1o alcohol CH2Cl2
Aldehyde
A2. Alkenes with at least one vinylic hydrogen undergo
oxidative cleavage when treated with ozone.
1. ozone
1-methylcyclohexene -----------------> 6-oxoheptanal
2. Zn, HAc
A3. Esters are partially reduced by diisobutylaluminum
hydride (DIBAH), normally at -78oC (dry-ice temperature) in
toluene.
1. DIBAH, toluene, -78oC
R-CO2R’ ---------------------------------->
RCHO
Ester
2. H3O+
Aldehyde
B. Ketone Synthesis
B1. Secondary alcohols are oxidized by a variety of
reagents such as PCC.
PCC
R-CHOH-R --------------> R-CO-R
2o alcohol
CH2Cl2
Ketone
B2. Ozonolysis of alkenes yields ketones if one of
the unsaturated carbon atoms is disubstituted.
1. ozone
CR2=CH2 ----------------> R-CO-R
+ H2CO
2. Zn, HAc
B3. Aryl ketones are prepared by Friedel-Crafts acylation
of an aromatic ring with an acid chloride in the presence of AlCl3.
ACl3
Ar +
RCOCl
-----------> Ar-CO-R
Aromatic Acid Chloride
Heat
B4. Methyl ketones are prepared by hydration of terminal
alkynes in the presence of Hg2+.
H3O+
R-C=CH ------------> RCOCH3
Alkyne
HgSO4 Methyl
Ketone
B5. Certain carboxylic derivatives can be converted
to ketones by using such reagents as dimethylcopperlithium reacting with
acid chlorides.
R-COCl + (CH3)2CuLi
--------------------> RCOCH3
Acid chloride Dimethyl copper
lithium
Ketone
Rxns of Aldehydes and Ketones
A. Oxidation of Aldehydes: Aldehyde + [O] -----> carboxylic
acid
[O] = HNO3, KMnO4, Jones reagent
[CrO3 in aqueous acid], Tollens reagent [Ag2O in
aqueous ammonia].
CrO3, H3O+
RCHO
--------------------> RCO2H
Aldehyde
Acetone, 0oC Carboxylic acid
Tollens reagent leaves C=C bonds and other functional
groups.
AgO2
PhCHO ------------------------>
PhCO2H
Aldehyde NH4OH, H2O,
EtOH Carboxylic acid
B. Oxidation of Ketones: Ketone + [O] ----> NR
Ketones are inert to most oxidizing agents but undergo
a slow cleavage to carboxylic acids reaction when treated with hot alkaline
KMnO4.
1. KMnO4, NaOH(aq), Heat
Acetone -----------------------------------> 2 Acetic
acid
2. H3O+
1. KMnO4, NaOH(aq), Heat
Cyclohexanone -----------------------------------> Hexanedioic
acid
2. H3O+
C. Nucleophilic Addition Rxns of Aldehydes and
Ketones
C1. Nucleophilic Addition of Water: Hydration
>C=O + water -----> gemdiol
Rxn favors starting materials.
C2. Nucleophilic Addition of HCN: Cyanohydrins
RCHO + HCN ---> RCH(OH)CN (cyanohydrin)
Nitriles (RCN) can be reduced with LiAlH4 to
yield primary amines
1. LiAlH4, THF
RCH(OH)CN --------------------------> RCH(OH)CH2NH2
2. H2O
and can be hydrolyzed by hot aqueous acid to yield carboxylic
acids.
H3O+, Heat
RCH(OH)CN -----------------> RCH(OH)CO2H
C3. Nucleophilic Add’n of Grignard & Hydride Reagents:
Alcohol Formation
C3a. Formaldehyde + Grignard -----> 1o
alcohol
1. CH3MgCl, Et2O
H2CHO -----------------------> CH3CH2OH
2. H3O+
C3b. Aldehyde + Grignard -----> 2o alcohol
1. PhMgCl, Et2O
CH3CHO -----------------------> CH3CH(OH)Ph
2. H3O+
C3c. Ketone + Grignard -----> 3o alcohol
1. PhMgCl, Et2O
CH3COCH3 ----------------------->
(CH3)2C(OH)Ph
2. H3O+
C3d. >C=O + LiAlH4 or NaBH4 ----->
alcohol
Reducing agents such as lithium aluminum hydride (LiAlH4),
sodium borohydride (NaBH4), or hydrogen and a catalyst.
Benzaldehyde + LiAlH4 ---> Benzyl alcohol
Cyclohexanone + NaBH4 ---> Cyclohexanol
Pt
Acetaldehyde + H2 ----> Ethanol
C4. Nucleophilic Addition of Amines: Imine and Enamine
Formation
>C=O + 1o amine -----> Imine
>C=O + 2o amine -----> Enamimine
Net result is the substitution of O by N of the amine.
Primary amines, RNH2, react with aldehydes
and ketones to yield imines, RN=C(R)2.
Hydroxylamine, NH2OH, yield oximes
Semicarbazide, NH2NHCONH2, yield
semicarbazones
2,4-dinitrophenylhydrazine yield 2,4-dinitrophenylhydrazones
(2,4-DNP's)
Secondary amines, R2NH, react to yield enamines,
R2N-C=C<
C5. Nucleophilic addition of Hydrazine: The Wolff-Kishner
Reaction
Converts aldehydes or ketones to alkanes
H2NNH2, KOH
>C=O --------------------------> >CH2
C6. Clemmensen reduction accomplishes the same
using amalgamated zinc, Zn(Hg), and concentrated HCl. Used when starting
materials are sensitive to base.
Zn(Hg)
>C=O ---------------> >CH2
HCl, Heat
C7. Nucleophilic Addition of Alcohols: Acetal Formation
>C=O + 2 ROH -----> >C(OR)2 acetal
One mole of carbonyl plus 2 moles of alcohol.
Rxn of carbonyl with ethylene glycol (HOCH2CH2OH)
yields a cyclic acetal.
Acetals are useful because they can serve as protecting
groups for aldehydes and ketones in the same way that trimethylsilyl ethers
serve as protecting groups for alcohols.
C8. Nucleophilic Addition of Phosphorous Ylides: The
Wittig Reaction
Ketones and aldehydes are converted to alkenes by reaction
with a phosphorus ylide, R2C--P+(C6H5)3.
>C=O + (R)2C--P+(C6H5)3
-----> >C=C(R)2
The net result is replacement of the carbonyl oxygen
atom by the R2C= group.
D. The Cannizzaro reaction
Aldehydes with no a
hydrogens (i.e. formaldehdye and benzaldehydes) when heated with
hydroxide yield one equivalent of carboxylic acid and one equivalent of
alcohol.
1. OH-
2 PhCHO -------------> PhCO2H
+ PhCH2OH
2. H3O+
E. Conjugate Nucleophilic
Addition to a,b-Unsaturated
carbonyl Groups
The net effect is addition of the nucleophile to the
b-carbon
of the C=C double bond, with the carbonyl group itself unchanged.
E1. Conjugate Addition of Amines
Primary and secondary amines
add to a,b-unsaturated
carbonyl compounds to yield b-amino
carbonyl compounds.
E2. Conjugate Addition of Alkyl Groups: Organocopper
Reactions
Conjugate addition of an
alkyl group to a,b-unsaturated
ketone is carried out by treating the ketone with lithium diorganocopper
reagent (Gilman reagent). Primary, secondary, and even tertiary alkyl groups
work as do aryl and alkenyl groups.
Spectroscopic Analysis of Ketones and Aldehydes
IR
Aldehydes and ketones show a strong C=O bond absorption
in the infrared region from 1660 to 1770 cm-1.
Aldehydes show two characteristic C-H absorptions in
the infrared region from 2720 -2820-1.
NMR
Aldehydic protons – 10d
There is spin-spin coupling of the aldehydic proton.
a-carbon hydrogens are deshielded
and absorb 2.0-2.3d.
methyl ketones show a characteristic three-proton singlet
near 2.1d.
Mass Spectroscopy
Aliphatic aldehydes and ketones that have hydrogens on
the gamma (g) carbon atoms undergo a spectral
cleavage (McLafferty rearrangement.)
Nature of The Carboxyl Group
The functional group present in organic acids is known
as the carboxyl group.
The carboxyl group can be symbolized as -COOH or -CO2H.
Nomenclature
Acids derived from open chain alkanes: replace -e with
-oic acid.
Acids with -COOH bonded to a ring: use the suffix -carboxylic
acid.
Structure
Systematic Name
Common Name
HCO2H
Methanoic acid
Formic acid
CH3CO2H
Ethanoic acid
Acetic acid
CH3CH2CO2H
Propanoic acid
Propionic acid
C6H4CO2H
Benzoic acid
Structure and Physical Properties of Carboxylic
Acids
Strongly associated due to hydrogen bonding.
Carboxylic acids with more than six carbons are only slightly
soluble in water.
Alkali metal salts are generally quite water soluble.
For most carboxylic acids, Ka = approximately
10-5. Acetic acid Ka = 1.76 x 10-5.
Ka values near 10-5 mean
only about 0.1% of the molecules in a 0.1 M solution are dissociated as
opposed
to the 100% dissociation found with strong mineral acids
such as HCl.
Stabilization of carboxylate anion increases acidity and
vice versa.
Electron withdrawing groups (e.g. halogen) (EAS deactivators)
increase acidity.
Electron donating groups (e.g.-R,-OR) (EAS activators)
decrease acidity.
Synthesis of Carboxylic Acids
A. Oxidation of substituted alkylbenzenes
(Ph-R) with KMnO4 or Na2Cr2O7
yield benzoic acids.
R = only 1o or 2 o.
3 o groups are not affected.
1. KMnO4, OH-, Heat
Ph-R ----------------------------->
Ph-CO2H
2. H3O+
B. Alkenes with at least one vinylic hydrogen are
oxidatively cleaved with KMnO4 to produce carboxylic acids.
If the other carbon of the alkene has two hydrogens it produces carbon
dioxide.
1. KMnO4
RCH=CH2 -------------------->
R-CO2H + CO2
2. H3O+
C. Oxidation of 1o alcohols and aldehydes.
Alcohols by Jones' reagent (CrO3, H2O, H2SO4).
Aldehydes by Jone’s or Tollens' reagent.
CrO3, H2O,
RCH2-OH ------------------>
RCO2H
H2SO4
CrO3, H3O+
RCHO -----------------------> RCO2H
Acetone, 0oC
D. Hydrolysis of nitriles by either hot aqueous acid or base.
H3O+, Heat or OH-,
Heat
R-CN ----------------------------------------> RCO2H
E. Carboxylation of Grignard reagents
1. CO2, Et2O
RMgX -----------------> RCO2H
2. H3O+
Reactions of Carboxylic Acids
A. Deprotonation
Acid + Base ----> Salt
The sodium and potassium salts of long-chain acids (fatty
acids) are referred to as soaps.
RCO2H + NaOH -----> RCO2-Na+
+ H2O
B. Reduction
Reduction by LiAlH4, THF, and Heat yields
primary alcohols.
1. LiAlH4, THF, Heat
RCO2H ------------------------------> RCH2OH
2. H3O+
Borane in tetrahydrofuran (BH3/THF) at room
temp. Nitro groups or carbonyls are not reduced by this method.
1. BH3, THF
RCO2H ------------------------> RCH2OH
2. H3O+
(NaBH4 does not reduce carboxylic acids.)
Suggested problems for Chapter 20
Carboxylic Acids
20.17; 20.19; 20.20; 20.22; 20.23;
20.24; 20.29; 20.31; 20.32; 20.34