Ethanol
2-Propanol
2-Methyl-2-butanol
I. Alcohols & Phenols
The functional group present in an alcohol or phenol
is the hydroxyl group (-OH). An alcohol can be understood
as an alkylated water, that is a carbon chain (R-) is substituted for a
hydrogen atom on water. A phenol substitutes an aromatic
ring (Ar-) for one hydrogen atom on water.
| Water | Alcohol | Methanol | Ethanol | Phenol |
| H-OH | R-OH | CH3-OH | CH3-CH2-OH | Ar-OH |
Naming Alcohols and Phenols
Alcohols are classified depending upon the number of
organic groups (R-) bonded to the hydroxyl bearing carbon.
| Type of Alcohol | No. of R- groups | General Formula | Example |
| Primary (1o) | 1 | CH3CH2-OH
Ethanol |
|
| Secondary (2o) | 2 | 2-Propanol |
|
| Tertiary (3o) | 3 | 2-Methyl-2-butanol |
IUPAC rules for naming alcohols
1. Identify the longest continuous chain of carbons
in which the hydroxyl group is attached to one of the carbons in the chain.
2. Number the chain so that the hydroxyl group
is attached to the lowest numbered carbons. (i.e., the –OH has priority
even over halo groups.)
3. Identify and locate the other branches on the
chain so that they are named alphabetically and their carbon number is
hyphenated onto the front of the name. If more than one of the same group
is present use the Greek prefix attached to the branch name. (2 = di, 3
= tri, etc.)
4. After all the branches have been named and located
then attach the carbon number that is attached to the hydroxyl group onto
the alkane name associated with the number of carbons found in the continuous
chain in step 1.
5. Drop the "e" on the alkane, alkene, or alkyne
name and attach the characteristic IUPAC ending "ol" to the rest of the
alkane, alkene, or alkyne name.
6. For polyhydroxy alcohols, locate two or more
carbons bearing the hydroxyl groups and hyphenate those carbon numbers
to the front of the alkane name and attach "diol" if two are involved,
"triol" if three hydroxyl groups are involved, etc.
7. If an alkene and an alkyne also contains the
-OH group, the name is expressed as:
(alkene) ___-en
___ol [first blank gives position of double bond, second blank
the position of -OH group]
(alkyne) ___-yn
___ol [first blank gives position of triple bond, second blank the
position of -OH group]
| methanol CH3-OH | 2-propanol |
| 2-methyl-1-butanol |
2-methyl-2-butanol |
| 2-phenylethanol |
cyclohexanol |
| 2-propen-1-ol |
3-butyn-2-ol |
The common names for alcohols are derived from the alkyl group corresponding to the parent compound.
|
|
CH3CH2OH |
|
Nomenclature of Phenols
If the hydroxyl group of a phenol is the principal group,
it is given the number 1, and the compound is named as a substituted phenol.
Phenols containing one substituent other than the –OH group can be named
by either ortho, meta, para system or by number. Phenols containing
more than one substituent are named by number.
Carboxyl and acyl groups have priority over the phenolic hydroxyl group.
Properties of Alcohols and Phenols
An alcohol molecule can be compared to a water molecule.
In an alcohol molecule, the hydroxyl oxygen and the two atoms bonded to
it are all in the same plane and have a bond angle of approximately 104o.
The bond angle in alcohols is approximately the tetrahedral value of 109o.
The oxygen is sp3 hybridized. The oxygyen-hydrogen bond
of an alcohol is the same as the oxygen-hydrogen bond length of water.
The hydroxyl group is polar.
There is a partial negative charge (d-) on the
oxygen atom and a partial positive charge (d+)
on the hydrogen of the hydroxyl group.
The ability of the hydroxyl group to hydrogen bond explains the higher boiling point of the alcohol when compared to its alkane parent or the analogous chloroalkane. The intermolecular attraction is higher in the alcohol so that the temperature must be raised to boil the substance. Other factors being equal, hydrogen-bonded molecules have higher boiling points than molecules that are not.
Recall that a hydrogen bond is an intermolecular attraction
in which a hydrogen atom bonded to a small electronegative atom (normally
N, O, or F) is attracted to an unshared electron pair on another small
electronegative atom.
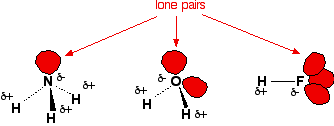
Lone pairs on N, O, and F. |
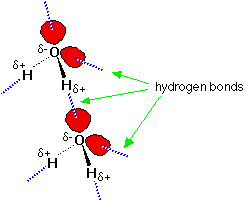
Hydrogen bonding between water molecules. |
|
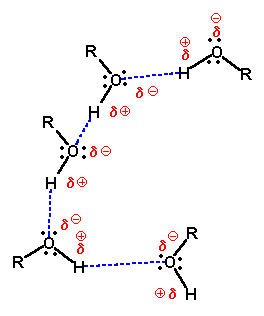
Hydrogen bonding between alcohol molecules. |
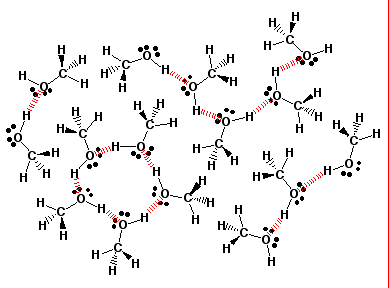
Hydrogen bonding network in methanol. |
|
Solubility Rule of Thumb: An organic compound is generally miscible with water if the carbon to oxygen ratio in the compound does not exceed 4:1. Methanol, CH3OH, is completely miscible, while 1-decanol, CH3(CH2)8CH2OH, is immiscible.
Acidity and Basicity of Alcohols and Phenols
Alcohols are amphoteric (amphiprotic) that is they can
act as a weak base or as a weak acid. Alcohols accept protons from
stong acids to yield an oxonium ion, ROH2+.
Alcohols undergo a slight dissociation in dilute aqueous solution by donating
a proton to water to yield an alkoxide ion, RO-.
Simple alcohols are about as acidic as water. The
pKa values of most alcohols are of the order of 18 (Ka
= 10-18). Water has a pKa value of about 16
(Ka = 10-16). Recall that as pKa values decrease
(Ka values increase) the acidity increases. Substituent groups can
have a significant effect.
| Relative Acidity
MeOH > H2O > ROH > RC=CH > H2 > NH3 > RH Except for methanol, most alcohols are slightly less acidic than water. |
Alcohols are much weaker acids than carboxylic acids or mineral acids and do not react with weak bases (e.g., amines or bicarbonate ion , and react only slightly with strong bases (e.g., NaOH).
Water reacts with alkali metals to produce alkali metal
hydroxide and hydrogen.
2 H-OH + 2 Na ----> 2 NaOH + H2
Alcohols react with alkali metals to produce alkali metal
alkoxide and hydrogen.
2 R-OH + 2 Na ----> 2 NaOR + H2
Alcohols also react with strong bases such as sodium hydride
(NaH), sodium amide (NaNH2), and Grignard reagents (RMgX).
R-OH + NaH ----> Na1+ OR1- + H2
R-OH + R’MgX ----> R’-H + RO-MgX
Alkoxides are strong bases and are oftern used as reagents in organic synthesis.
The most characteristic property of phenols is their acidity. Phenols are weak acids but are about a million times more acidic than alcohols. For example, phenol has a pKa value of about 10 (Ka = 1 x 10-10 ) and ethanol has a pKa value of about 16 (Ka = 1 x 10-16 ). Due to there acidity, phenols are soluble in dilute aqueous NaOH.
The phenoxide ion is stabilized by delocalization of the negative charge on the oxygen to the ring.
Alkyl substitution of phenols produces negligible
changes in acidity. Only when the substituent is an electron-withdrawing
groups (i.e., nitor groups) is a substantial change in acidity noted.
Synthesis of Alcohols
A. Synthesis of alcohols from alkenes:
A1. Acid catalyzed hydration of alkenes
(Reversible, rearrangement products,
Markovnikov, limited to industrial prod.)
(Markovnikov's rule = "Them that has, get's!")

A2. Hydroboration of alkenes (syn stereochemistry, non-Markovnikov)
A3. Oxymercuration of alkenes ( regioselective - Markovnikov)
A4. Hydroxylation of alkenes to yield 1,2-diols (syn stereochemistry)
A5. Hydroxylation of alkenes to yield 1,2-diols (anti stereochemistry)
B. Synthesis of alcohols from reduction of carbonyl
compounds
B1. Reduction of aldehydes and ketones
Aldehydes are reduced to 1o alcohols and ketones
to 2o alcohols.
[H] = Sodium borohydride: (1) NaBH4/ethanol
(2) H3O+ or Lithium aluminum hydride:
(1) LiAlH4/ether (2) H3O+
Lithium aluminum hydride >>> reactive than Sodium
borohydride
B2. Reduction of carboxylic acids and esters
Sodium borohydride reacts very slowly
with esters and not at all with acids.
Lithium aluminum hydride: (1) LiAlH4/ether
(2) H3O+, is the reagent of choice.
Carboxylic acids and esters are reduced
to give primary alcohols.
C. Synthesis of alcohols from carbonyl compounds
reacting with Grignard reagents
Formaldehyde + Grignard ----- > 1o
alcohols
Aldehydes + Grignard ----- > 2o alcohols
Ketones + Grignard ----- > 3o alcohols
Esters + 2 Grignard ----> 3o alcohol (two R substituents come from the Grignard reagent)
Carboxylic acids don’t give addition products. Grignards
cannot be prepared from molecules containing -OH, -HN, -SH, -COOH functional
groups.
RCO2H + R'-MgX ------>
R-CO21- 1+MgX + R'H
Reactions of Alcohols
A. Alcohols react with alkali metals,
sodium hydride (NaH), or sodium amide (NaNH2) to produce alkoxide
ions.
Phenols react with sodium hydroxide to give the phenoxide ion.
Alkoxides and phenoxides are strong bases and are used as reagents.
B. Dehydration of alcohols to yield
alkenes
B1. 3o alcohol
+ H3O1+ ---> Alkene
Order of dehydration reactivity:
3o > 2o > 1o
Zaitsev’s rule is followed and the more substituted alkene is preferred product. (The poor get poorer.)
B2. Phosphorus oxychloride (POCl3)
in the basic amine solvent pyridine is less harsh and can effect
dehydration of secondary and tertiary at 0oC.
C. Conversion alcohols into alkyl halides
C1. Tertiary alcohol + HCl or HBr
at 0oC ------> alkyl halide
R-OH + HX---> R-X
Reactivity of ROH: allyl, benzyl >
3o > 2o > 1o
Reactivity of HX: HI > HBr > HCl
C2. Primary and secondary alcohols are best converted using SOCl2 or PBr3.
D. Conversion of alcohols into tosylates
and mesylates

Tosylates and mesylates are good leaving groups.
Nu:1- + RCH2-OTos ----> RCH2-Nu
+ -OTs1-
E. Oxidation of Alcohols
In organic terminology:
Oxidation
[O] is:
1. The addition of oxygen to a molecule.
2. The removal of hydrogen from a molecule.
Reduction
[H] is:
1. The removal of oxygen from a molecule.
2. The addition of hydrogen to a molecule.
| Oxidation of a primary alcohol yields an aldehyde or
carboxylic acid.
1o alcohol ------> Aldehyde [O]
|
| Oxidation of a secondary alcohol yields an ketone.
2o alcohol ------> Ketone |
| Tertiary alcohols do not undergo oxidation.
3o alcohol ------> NR |
Milder oxidation is accomplished by using pyridinium chlorochromate (PCC, C5H6NCrO3) in dichloromethane (CH2Cl2).

Secondary alcohols are oxidized to ketones using in Na2Cr2O7 in acetic acid.
Synthesis of Phenols
Phenols are rings with an -OH group directly bonded to
an aromatic ring, ArOH.
Phenol is the name of both a specific compound and of
a class of compounds.
A. Alkali fusion of aromatic sulfonates (limited
to alkyl-substituted phenols)
(1) SO3/H2SO4
Toluene
-----------------------> p-Methylphenol
(2) NaOH/300oC
(3) H3O1+
B. Hydrolysis of arenediazonium salts
(1) HNO2/H2SO4
2-Bromo-4-methylaniline --------------------------->
2-Bromo-4-methylphenol
(2) H3O1+
Reactions of Phenols
A1. Electrophilic Aromatic
Substitution Rxns of Phenols
The -OH group is a strongly activating, ortho- and para-directing substituent
in EAS reactions.
B. Oxidation of Phenols: Quinones
Treatment of a phenol with any of a number of oxidizing
agents yields 2,5-cyclohexadiene-1,4-dione (benzoquinone).
phenol + Fremy's salt [(KSO3)2NO] ---> Benzoquinone
Spectroscopy of Alcohols and Phenols
IR
O-H stretching absorption at 330-3600 cm-1
NMR
Carbon atoms bonded to electron-withdrawing –OH groups
are deshielded and absorb at a lower field in the C-13 NMR spectrum.
Most C-OH absorptions fall in the range of 50-80 d.
Hydroxyl hydrogens are also deshielded and occur in the range of 3.5-4.5 d.
D2O exchange will cause the –OH absorption to disappear.
An ether can be seen as a water molecule with both hydrogens
substituted by some R group (e.g. R = alkyl or aryl).
H-O-H R-O-R
Water Ether
Ethers are generally unreactive and often used as solvents.
Thiols R-SH (sulfur analogs of alcohols)
Sulfides R-S-R (sulfur analogs of ethers)
Ether Nomenclature
Two systems:
1. Simple ethers with no other functional groups
are named by identifying the two organic substituents and adding the word
ether.
a. symmetrical ethers have both groups identical.
Methyl ether or dimethyl ether = CH3-O-CH3
Phenyl ether = Ph-O-Ph
b. unsymmetrical ethers have different groups
Ethyl methyl ether = CH3-O-CH2-CH3
Phenyl propyl ether = Ph-O-CH2-CH2-CH3
2. If other functional groups are present or if
one group has no simple name, the compound may be named as a alkoxy derivative
(an alkoxy, RO-, is named as a substituent). These groups are never treated
as principal groups. The principal chain is the longest continuous chain
or, in case of chains of equal length, the most highly branched chain.
1-Ethoxyhexane = CH3CH2-O-CH2CH2CH2CH2CH2CH3
Structure, Properties, and Sources of Ethers
Bond angle in alcohol is approximately tetrahedral value
of (112o in dimethyl ether). The oxygen is
sp3 hybridized. Ethers are slightly
polar.
Synthesis of Ethers
A. The Williamson Ether Synthesis
In the Williamson ether synthesis, metal alkoxides react
with primary alkyl halides and tosylates by an SN2 pathway.
There are two variations of the Williamson ether
A1. Alcohols are reacted with a
strong base to produce the alkoxide ion, which then reacts with halide
or tosylate.
(1) NaH
R-OH
----------------------------------> R-O-R'
(2) R'X (1o halide or tosylate)
(1) NaH
t-Bu-OH ------------------>
t-Bu-O-CH3
(2) CH3-I
A2. Silver oxide, Ag2O,
can be used instead of the strong base. No need to generate metal
alkoxide intermediate.
Ag2O
t-Bu-OH + CH3-I
----------------> t-Bu-O-CH3
B. Alkoxymecuration of Alkenes
(CF3CO2)2Hg
(Mercuric trifluoroacetate)
Markovikov
addition of the alcohol to the alkene.
1. (CF3CO2)2Hg , Alcohol
Alkene -------------------------------------->
Ether
2. NaBH4
1. (CF3CO2)2Hg , EtOH
Cyclohexene
-------------------------------------> Cyclohexyl ethyl ether
2. NaBH4
Reactions of Ethers
A. Acidic Cleavage
Ether + HX -----> Alkyl halide + Alcohol
HX = HI or
HBr (HCl does not work.)
Halide attacks
at less hindered site to produce alkyl halide.
Ethyl phenyl ether + HBr/Heat/Water ----> Phenol + Bromoethane
Ethyl isopropyl ether + HI/Heat/Water -----> Isopropyl alcohol + Iodoethane
B. Reactions of Ethers: Claisen Rearrangement
Claisen rearrangement
is specific to allyl aryl ethers, Ar-O-CH2-CH=CH2.
Treatment of phenoxide ion with 3-bromopropene (allyl
bromide) results in a Williamson ether synthesis and production of an allyl
phenyl ether.
1. NaH/THF
Ar-OH -------------------------------->
Ar-O-CH2-CH=CH2 (Allyl phenyl ether)
2. Br-CH2-CH=CH2
Heating the allyl phenyl ether to 200-250oC
then effects Claisen rearrangement, leading to an o-allylphenol.
Epoxides
Common cyclic ethers
| THF (C4H8O) | 1,4-Dioxane (C4H8O2) |
|
|
Synthesis of Epoxides
A. Industrial ethylene oxide production:
Ethylene + Air/ Silver oxide ------ > Ethylene oxide
B. Alkene + peroxyacid, RCO3H ---- > Epoxide. [m-Chloroperoxybenzoic is the peroxyacid most commonly used.]
C. Alkene + Cl2/H2O ---> Halohydrin + NaOH/H2O -----> Epoxide + Water + NaCl
Ring-Opening Reactions of Epoxides
A. Acid-catalyzed
Epoxide +
HX/Ether -----> halohydrin [X bonds at least hindered
in carbon in 1o and 2o but most in 3o]
B. Base-catalyzed
B1a. Epoxide + Hydroxide ----->
diol [base attack at less hindered site]
B1b. Epoxide + Alkoxide -----> alkoxyalcohol [base attack at less hindered site]
B2. Epoxide + Grignard -----> alcohol with extended carbon chain
Crown Ethers
Crown ethers are cyclic polyethers.
Formula x-crown-y where x = total number of atoms in the ring and y is the number of oxygen atoms.

Crown ethers solvate metal cations by sequestering the metal in the center of the polyether cavity.
Result is a "naked" anion which can be carried into organic solution in aprotic conditions.
Spectroscopy of Ethers
Ethers are difficult to distinguish by IR.
NMR of carbon and protons are shifted downfield.
Thiols and Sulfides
Thiols are sulfur analogs of alcohols. R-SH
Thiol Nomenclature
The rules for naming thiols are exactly like those for
alcohols, except that the suffix -ol is replaced by -thiol. The –SH group
is itself is referred to as a mercapto group.
trans-2-Butene-1-thiol (North American skunk spray)
Sulfides are sulfur analogs of ethers.
R-S-R
Sulfide Nomenclature
Sulfides are named by following the same rules used for
ethers, with sulfide used in place of ether.
CH3-S-CH3
CH3CH2-S-CH2CH2CH3
Dimethyl sulfide
Ethyl propyl sulfide
Thiol Synthesis
A. Alkyl halide + hydrosulfide anion
------> Thiol
CH3-CH2-CH2-Br + NaSH
-------> 1-propanethiol
B. Best method is to treat an alkyl
halide with thiourea and then follow with hydrolysis conditions.
(1) Thiourea [CS(NH2)3]
Alkyl halide ------------------------------------>
Thiol
(2) NaOH, H2O
(1) Thiourea [CS(NH2)3]
CH3-CH2-CH2-Br ------------------------------------->
1-propanethiol
(2) NaOH, H2O
Thiol Reactions
Thiols are oxidized by bromine or iodine to yield disulfides,
RSSR.
I2
2 R-SH ---------------> R-S-S-R
thiol Zn, H+
disulfide
Sulfide Synthesis
Thiols react with strong bases to give the corresponding
thiolate anion, RS-. These ions react with 1o
or 2o halides to give sulfides.
(1) NaH
Thiol ---------------------------> Sulfide
(2) 1o or 2o Halide
(1) NaH
Ph-SH
-------------------> Ph-S-CH3
Benzene Thiol (2)
CH3I Methyl
phenyl sulfide
Sulfide Reactions
A. Dialkyl sulfides react with 1o
alkyl halides to produce trialkylsulfonium salts (R3S+).
THF
CH3-S-CH3 + CH3I
-------------> (CH3-)3S+I-
Trimethylsulfonium iodide
B. Sulfides can be oxidized to sulfoxides
(R2SO) and sulfones (R2SO2).
H2O2 , H2O
CH3O3H
Ph-S-CH3 ------------------------->
Ph-SO-CH3
-------------------> Ph-SO2-CH3
Methyl phenyl sulfoxide
Methyl phenly sulfone
Suggested problems
17.24, 17.25, 17.26, 17.27, 17.28, 17.29, 17.31,
17.32, 17.33, 17.34, 17.37, 17.38, 17.39, 17.47, 17.49,
17.54, 17.56, 17.57, 17.58, 17.59, 17.60, 17.61,
17.61, 17.62
18.25, 18.26, 18.27, 18.28,
18.29, 18.52, 18.53, 18.54