CHEMISTRY 122: SYNTHESIS of SOAP
| INTRODUCTION
Soap is produced by the saponification (hydrolysis) of a triglyceride
(fat or oil). (See Figure 1.) In this process the triglyceride is reacted
with a strong base such as sodium or potassium hydroxide to produce glycerol
and fatty acid salts. The salt of the fatty acid is called a soap.
Fatty acids are straight-chain monocarboxylic acids. The most
common fatty acids range in size from 10-20 carbons and most often have
an even number of carbon atoms including the carboxyl group carbon. The
carbon-carbon bonds in saturated fatty acids are all single bonds, while
unsaturated fatty acids have one or more carbon-carbon double bonds in
their chains. One example of a saturated fatty acid is palmitic acid, CH3-(CH2)14-CO2H.
Fatty acids are seldom found as free molecules in nature but are most
often a part of a larger molecule called a
triglyceride. Triglycerides
consist of a three-membered carbon chain (glycerol backbone) with a fatty
acid bonded to each of the three carbon atoms in the glycerol backbone.
The bond between the fatty acid and the glycerol backbone is referred to
as an ester linkage. In the saponification process the ester linkage is
broken to form glycerol and soap.
|
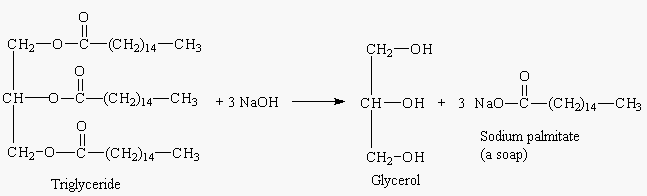 |
| Figure 1. Saponification of a triglyceride |
EXPERIMENTAL PROCEDURE FOR SYNTHESIS OF SOAP
| 1. PUT ON YOUR CHEMICAL SPLASH-PROOF SAFETY GOGGLES! |
| Caution: Oil will be hot, and may splatter or
catch fire. Wear goggles at all times. |
| Assembling the hot water bath |
2. Assemble a hot water bath by filling an 800 mL beaker approximately
3/4 full with water and begin heating the water with a bunsen burner.
3. Add three or four boiling chips to the water in the hot water bath
to prevent the water from boiling over.

| Preparing the reaction mixture |
4. In a 150 mL beaker add the following ingredients.
A. 15 mL of oil (or 10 g of solid shortening)
B. 20 mL of 20% NaOH
C. 10 mL of ethanol
D. 3 boiling chips (These will help prevent the mixture from boiling
over while it is being heating.)
5. Note the total volume (level) in the 150 mL beaker and how many layers
the ingredients initially form.
| Heating the reaction mixture |
6. Begin heating the reaction mixture by clamping the beaker and contents
in the hot water bath. Heat the mixture for about 25 minutes after
the water comes to a slow boil. The 150 mL beaker should be clamped so
that the reaction mixture is below the level of the water in the water
bath. Maintain the water level in the water bath by adding water as needed.
7. Using a stirring rod, stir the reaction mixture frequently so that
it does not boil over.
8. Maintain the total volume of the reaction mixture by adding small
quantities of 1:1 (volume/volume) ethanol-deionized water.
9. After the initial 25 minute heating there should be no separation
of layers in the beaker.
| Testing the reaction mixture |
10. Test the reaction mixture to determine if the saponification process
is complete by carefully placing a few drops of the reaction mixture in
a 6-inch test tube. Add 10 mL of cold water. If fat droplets form, add
5 mL of the 20% NaOH and 5 mL of ethanol to the beaker and continue to
heat for an additional 10 minutes, or until no fat droplets form upon testing.
| Isolating the soap CAUTION: Remember that
the beaker and clamp are hot! |
11. When the saponification process is complete, turn the bunsen burner
off, add 25 mL of deionized water to the beaker and place the beaker on
the bench top to cool for about 5-6 minutes. Then place the soap reaction
beaker into an ice bath and cool for about 10 minutes.
12. At this point, measure out about 50 mL of saturated NaCl solution
and cool it also for about 5-6 minutes in the ice bath.
13. After the cooling time is complete for the soap reaction mixture,
decant any liquid from the beaker. (Be careful not to pour off the soap.)
14. Next add, add the 50 mL cold, saturated NaCl solution to the soap beaker
and stir thoroughly with a glass rod. This process separates the soap from
the glycerol and excess base and is called "salting out."
15. Collect the solid soap, using a Buchner funnel. [Note: Decant as much liquid
off before adding the solid soap to the Buchner funnel.]
16. While the air is being drawn over the soap in the funnel, wash the
soap with two 20 mL volumes of ice cold deionized water. Continue to draw
air over the soap for another 3 minutes.
17. Fill four, 6-inch test tubes one-third full with deionized water. Place
a small amount of the soap in each test tube and mix the soap and water
well.
A. Test the pH of the first tube by dipping a clean stirring rod into
the solution and touching the solution to both red and blue litmus paper.
Record your observations on the Data Sheet.
B. In the second test tube add several drops of calcium chloride solution.
Record your observations.
C. In the third test tube add several drops of iron(III) chloride solution.
Record your observations.
D. In the fourth test tube add a single drop of kerosene and shake the
solution. Record your observations.
18. Pour the contents of the four test tubes into the designated waste
container.
19. Place the soap in the designated container.
20. Thoroughly rinse all of the glassware with water before storing.
| CHEMISTRY 122: DATA SHEET
FOR SYNTHESIS OF SOAP |
| Name __________________________ |
Hood No. _________________ |
Date _________________ |
| PUT ON YOUR CHEMICAL SPLASH-PROOF
SAFETY GOGGLES! |
| Show
all calculations. |
SOAP ANALYSIS
| TEST TUBE # 1 |
OBSERVATION |
| Red Litmus Paper |
|
| Blue Litmus Paper |
|
What explanation can you give for the observed color changes
of the litmus paper?
|
| TEST TUBE # 2 |
OBSERVATION |
| Calcium chloride test
|
|
| TEST TUBE # 3 |
OBSERVATION |
| Iron(III) chloride test
|
|
| TEST TUBE # 4 |
OBSERVATION |
| Kerosene test
|
|
|

