
Aspirin (acetylsalicylic acid) is a synthetic organic derived from salicylic acid. Salicylic acid is a natural product found in the bark of the willow tree and was used by the ancient Greeks and Native Americans, among others, to counter fever and pain. However, salicylic acid is bitter and irritates the stomach.
A German chemist named Felix Hoffman is credited with being the first to synthesize aspirin in 1897. Hoffman's father had severe arthritis but could not tolerate salicylic acid he was taking for pain relief. The name given for Hoffman's new compound was A-spirin. Apparently this comes from acetylation (A-), together with Spirin, part of the name for Meadow-sweet (Spiraea ulmaria), a plant rich in salicylates.
Friedrich Bayer, the employer of Hoffman, patented the name and began marketing the product in 1899. It was a huge success and sales grew rapidly. Bayer's company set up by himself, is generally reckoned to have been the first pharmaceutical company, and the production of aspirin is generally accepted to have laid the foundation of the modern pharmaceutical industry.
In this experiment you will synthesize aspirin (acetylsalicylic acid, C9H8O4 ), purify it, and determine the percent yield. The purity of the product will be confirmed by qualitative analysis and by measuring its melting point range.
The reaction that is used for the synthesis is shown below. In this reaction, an excess of acetic anhydride (C4H6O3) is added to a measured mass of salicylic acid (C7H6O3) in the presence of a catalyst, sulfuric acid (H2SO4). The mixture is heated to form the acetylsalicylic acid (C9H8O4) and acetic acid (C2H4O2). After the reaction takes place, water is added to destroy the excess acetic anhydride and cause the product to crystallize. The aspirin is then collected, purified by recrystallization, and its melting temperature measured.

| SAFETY CONSIDERATIONS
Wear goggles throughout this experiment. This experiment uses salicylic acid, acetic anhydride and phosphoric acid. The salicylic acid and aspirin may cause irritation to your skin or eyes, but are basically not hazardous. An excess of these can be disposed of in the sink or, if packaged, in the trash. If you spill some, wipe it up with a wet paper towel and throw the towel in the trash. The acetic anhydride and sulfuric acid can cause bad burns. Use them only in the hood and be sure the hood fan is on! Wear gloves when using these chemicals. Excess chemicals must be disposed of in the plastic tub of water. This will convert the acetic anhydride to vinegar and dilute the sulfuric acid. If you spill a lot of either of these, notify your instructor. |
| 1. PUT ON YOUR CHEMICAL SPLASH-PROOF SAFETY GOGGLES! |
| 2. Adding The Starting Materials |
B. Using the graduated cylinder located under the hood, measure out 7.00 mL of acetic anhydride and add this to the flask. Be sure to do this in the hood and wearing your goggles. Don't let the acetic anhydride contact your skin and don't get the vapors in your eyes.
C. Carefully add 8 drops of concentrated sulfuric acid (18 M H2SO4),
a catalyst, to the flask.
| 3. Heating The Starting Materials |
B. Place the flask in the water bath and heat. After the water
begins to boil, heat for an additional 15 minutes. (NOTE: The hot water
bath will be used again later in the procedure.)
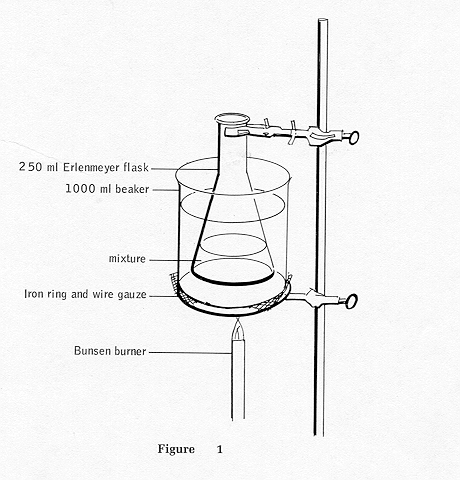 |
| 4. Cooling The Reaction Mixture |
B. After the flask has cooled for about 3 minutes, CAUTIOUSLY add 15 mL of room temperature water to the flask to facilitate the decomposition of the excess acetic anhydride. Swirl the flask to mix the contents.
C. Label your flask containing the reaction mixture and place it in
an ice bath and cool until the crystallization of the aspirin appears
complete (approx. 15 min.). If crystals do not appear, you can scratch
the walls of the flask with a stirring rod to induce crystallization.
| 5. Isolating The Product |
B. Rinse the flask twice with 3 mL of ice cold water to remove any residual crystals.
C. Discard the filtrate left in the filter flask into the waste container
under the hood.
 |
| 6. Recrystallizing The Aspirin |
B. Add 10 mL of 95% ethanol to the beaker and if necessary, warm (do not boil!) the mixture in the water bath to dissolve the crystals. If the crystals do not all dissolve, add 2 mL more of the ethanol and continue to warm the mixture to dissolve the crystals.
C. When the crystals are all dissolved, add 10 mL of deionized water, cover the beaker with a watch glass, and allow the solution to cool slowly on the lab bench undisturbed for about 10 minutes.
D. After the 10 minutes cooling on the lab bench, complete the crystallization
by placing the beaker and contents in the ice bath. (Label your beaker!)
Crystals should form. If an "oil" appears instead of a solid, reheat the
beaker in the hot water bath until the oil disappears. If crystals do not
appear, you can scratch the bottom of the beaker with a stirring rod to
induce crystallization.
| 7. Drying The Purified Aspirin |
B. Dry the crystals by pulling air through them for about 15 minutes. (Discard the filtrate left in the filter flask into the waste container under the hood. Rinse the filter flask with water and discard the rinse water into the waste container under the hood.)
C. Place the aspirin onto a doubled piece of paper towel and set aside
to dry while performing the qualitative analysis of the aspirin. (Wash
the filter funnel with water and discard the rinse water into the waste
container under the hood.)
| 8. Analyzing the Aspirin Quality |
1. Add about 1 mL of deionized water to five clean 10-cm test tubes. Using a clean stirring rod, place a crystal of salicylic acid into the first test tube. In the second, place a crystal of powdered commercial aspirin, and in the third, place a crystal of your synthesized aspirin. The forth test tube is the control.
2. To each test tube add 1 drop of iron(III) chloride) solution. Shake each test tube and observe the colors produced. Record your observations and conclusions on the Data Sheet.
B. Measure the melting point range of your synthesized aspirin with
the Meltemp Apparatus as demonstrated by your lab instructor and compare
to the value for pure aspirin of 138-140 oC. Record the melting
temperature on the Data Sheet.
| 9. Calculating the Percent Yield of Aspirin |
B. Based on your percent yield, iron(III) chloride test, and the measured melting point range, draw a conclusion about the success of your synthesis.
C. Place your aspirin in the jar labeled "Student Prep Aspirin".
IRON (III) CHLORIDE TEST
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||