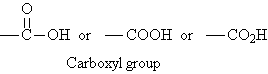
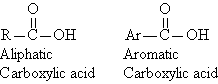
Carboxylic acids are a family of organic compounds
characterized by the presence of the carboxyl group. The term "carboxyl"
is a contraction of "carbonyl" and "hydroxyl".
 |
 |

Carboxylic acids are weak acids. In general, the
dissociation of a carboxylic acid can be represented as:
RCO2H(aq) <====> RCO21-(aq)
+ H1+(aq)
In other words, carboxylic acids do not completely dissociate (typically less than 5%) into the carboxylate ion and the hydronium ion. For example, in a 1.0 molar solution of acetic acid, the acetic acid is only about 0.5% dissociated.
HC2H3O2(aq) <======>
Hl+(aq) + C2H3O21-(aq)
A strong acid, such as hydrochloric acid, completely
dissociates (100%) in aqueous solution: HCl(aq) -----> Cl1-(aq)
+ H1+(aq)
Note: The hydronium ion can be indicated as H1+(aq) or H3O1+.
Like the carbonyl of aldehydes and ketones, the carboxyl group is polar.
The carboxyl group can hydrogen bond with water and with other carboxyl groups. Because of hydrogen bonding, carboxylic acids form dimers. Most carboxylic acids exist as dimers. Boiling points of carboxylic acids are higher than the alcohols with the same number of carbon atoms.
Carboxylic acids follow the rule of thumb solubility guideline. In other words, carboxylic acid molecules that do not exceed the ratio of 4 carbon atoms for every 1 oxygen atom are soluble in water.
Carboxylic acids are characterized by sharp, sour tastes and unpleasant odors.
Long chain carboxylic acids are referred to as fatty acids. Normally this includes carboxylic acids with carbon chains of 10 or more atoms.
Carboxylic acid derivatives include esters, acid chlorides, acid anhydrides, and amides.

IUPAC Nomenclature of Carboxylic Acids (Systematic
Nomenclature)
Determine the parent compound, the longest continuous
carbon chain bearing the carboxyl group.
1. Replace -e with -oic acid.
If there are two carboxyl groups, the suffix -dioic is used.
2. Number the chain so that
the carboxyl carbon is carbon number 1.
3. Name and number substituents
in the usual way.
In the common system of nomenclature, names are derived from Latin or Greek words. Substituted carboxylic acids are named as derivatives of the parent compound with Greek letters used to indicate the position of the substituent.
 |
Aromatic carboxylic acids are usually named, in either
system, as derivatives of benzoic acid.
Generally, the -oic acid or -ic acid suffix is attached
to the appropriate prefix.
Benzoic acid 
IUPAC and Common Names of Several Carboxylic Acids
| Structure | Systematic Name | Common Name | Source |
| HCO2H | Methanoic | Formic | Found in ant venom. |
| CH3CO2H | Ethanoic | Acetic | Found in vinegar (4 to 5%). |
| CH3CH2CO2H | Propanoic | Propionic | Swiss cheese |
| CH3(CH2)2CO2H | Butanoic | Butyric | Rancid butter |
| CH3(CH2)3CO2H | Pentanoic | Valeric | |
| CH3(CH2)4CO2H | Hexanoic | Caproic | |
| CH3(CH2)6CO2H | Octanoic | Caprylic | |
| CH3(CH2)8CO2H | Decanoic | Capric | |
| CH3(CH2)10CO2H | Dodecanoic | Lauric | Saturated fatty acid |
| CH3(CH2)12CO2H | Tetradecanoic | Myristic | Saturated fatty acid |
| CH3(CH2)14CO2H | Hexadecanoic | Palmitic | Saturated fatty acid |
| CH3(CH2)16CO2H | Octadecanoic | Stearic | Saturated fatty acid |
| CH3-(CH2)7-CH=CH-(CH2)7-CO2H | Oleic acid | Unsaturated fatty acid | |
Dicarboxylic Acid Common Names
| Structure | Common Name | Mnemonic |
| HO2CCO2H | Oxalic | Oh |
| HO2CCH2CO2H | Malonic | My |
| HO2C(CH2)2CO2H | Succinic | Such |
| HO2C(CH2)3CO2H | Glutaric | Good |
| HO2C(CH2)4CO2H | Adipic | Apple |
| HO2C(CH2)5CO2H | Pimelic | Pie |
| HO2C(CH2)6CO2H | Suberic | Sweet |
| HO2C(CH2)7CO2H | Azelaic | As |
| HO2C(CH2)8CO2H | Sebacic | Sugar |
Aromatic Dicarboxylic Acids
| Phthalic
|
Isophthalic
|
Terephthalic
|
Common Carboxylic Acids [Note: The following information was taken from http://www.bartleby.com]
Citric acid is a colorless translucent crystalline acid, C6H8O7, principally derived by fermentation of carbohydrates or from lemon, lime, and pineapple juices and used in preparing citrates and in flavorings and metal polishes.
Lactic acid is a syrupy, water-soluble liquid, C3H6O3, produced in muscles as a result of anaerobic glucose metabolism, and present in sour milk, molasses, various fruits, and wines. A synthetic form of the compound is used in foods and beverages as a flavoring and preservative, in dyeing and textile printing, and in pharmaceuticals.
Adipic acid is a white crystalline dicarboxylic acid, C6H11O4, that is derived from oxidation of various fats, slightly soluble in water and soluble in alcohol and acetone, and used especially in the manufacture of nylon and polyurethane foams.

Reactions and Preparation of Carboxylic Acids
1. Preparation by oxidation of primary alcohols and aldehydes.
[O]
[O]
R-CH2-OH -----> RCHO ---->
RCO2H
1o alcohol
aldehyde acid
It is difficult to stop the oxidation of a primary alcohol
to the aldehyde.
2. Acid-Base Reactions / Neutralization

The reaction between a strong base and a carboxylic acid
is called a neutralization.
The products of such a reaction are the carboxylate metal
salt and water.
Acetic acid + Sodium hydroxide ---> Sodium acetate + Water
3. Esterification
Carboxylic acids react with alcohols
to produce esters.
RCO2H + ROH ----> RCO2R + H2O
Esters
Esters are carboxylic acid derivatives that have the
general formula of RCO2R.
Esters generally have pleasant odors and are found in
nature.
Ester Nomenclature
1. Identify and name the alkyl group attached to
the oxygen of the carboxyl group
2. Use the name of the carboxylic acid, with the
-ic ending replaced with -ate.
Methyl acetate
CH3CO2CH3 or CH3COOCH3
Ethyl acetate
CH3CO2CH2CH3 or CH3COOCH2CH3
Ethyl butanoate
CH3CH2CH2CO2CH2CH3
Methyl benzoate
PhCO2CH3
Dimethyl malonate
CH3OOCCH2COOCH3
Reactions Involving Esters
1. Preparation of Esters
Acid + Alcohol <---> Ester + Water
RCO2H + R'OH ---> RCO2R'
2. Hydrolysis
Esters are hydrolyzed, either by aqueous acid or aqueous
base.
Under acidic conditions, the carboxylic acid and alcohol
are produced.
RCO2R' + H3O1+
----> RCO2H + R'OH
Under basic conditions, the salt of the acid and the
alcohol are produced.
RCO2R' + NaOH ----> RCO21-Na1+
+ R'OH
Base hydrolysis is called saponification.
A soap is a sodium or potassium salt of a fatty
acid. A fatty acid is a long chain carboxylic acid containing 10-22 carbon
atoms. The role of soap in the removal of soil and grease is best
understood by considering the functional groups in soap molecules.
The long, continuous hydrocarbon side chains in a soap molecule resemble
an alkane, and they dissolve other nonpolar compounds such as oils and
greases. This part of the molecule is referred to as hydrophobic,
which means "water-hating." This part of the molecule is repelled by water.
The carboxylate end of the molecule is called hydrophilic, which means
"water-loving." The hydrophobic end can also be viewed as being lipophilic
that is oil-soluble. The hydrophilic end can be viewed as being lipophobic
that is oil-insoluble. When soap is dissolved in water, the carboxylate
end actually dissolves. The hydrocarbon part is repelled by the water
molecule so that a thin film of soap is formed on the surface of the water
with the hydrocarbon chains protruding outward. This greatly lowers the
surface tension of the water. When soap solution comes in contact with
oil or grease, the hydrocarbon part dissolves in the oil or grease, but
the polar carboxylate group remains dissolved in water. When particles
of oil or grease are surrounded by soap molecules, the resulting "units"
formed are called micelles. Micelles repel one another because they
are surrounded by the negatively charged carboxylate ions. Mechanical action
(scrubbing or washing machine tumbling) causes oil and grease to be surrounded
by soap molecules and broken into small droplets so that relatively small
micelles are formed. These small micelles are then washed away. Careful
examination of this solution shows that it is an emulsion containing suspended
micelles.
Acid Chlorides
Acid chlorides are named by dropping the -ic of the acid
name and replacing it with -oyl chloride.
CH3COCl is acetyl chloride
and PhCOCl is benzoyl chloride.
Acid chlorides are produced by reacting an acid with thionyl
chloride.
RCO2H + SOCl2
---> RCOCl
Acid chlorides react violently with water to produce the
parent acid and HCl.
RCOCl + Water -----> Acid + HCl
Acid Anhydrides
Acid anhydrides are named by replacing acid with anhydride.
Anhydrides are prepared by reacting the carboxylic acid with an acid chloride.
![]()
Phosphoesters and Thioesters
Alcohols react with phosphoric acid to produce a phosphate
ester, or phosphoester.

Suggested Problems
15.15, 15.16, 15.24, 15.29, 15.31, 15.37, 15.39