The term "carbohydrate" comes from the observation that their apparent molecular formula was Cn(H2O)n. For example, in the case of glucose, the molecular formula of C6H12O6 can be understood as C6(H2O)6.
Carbohydrates can be classified according to size (i.e., the number of sugar units per molecule). The term "saccharide" (derived from Latin for sugar) is the chemical name for a sugar unit. A monosaccharide is composed of one simple sugar unit. A disaccharide is composed of two simple sugar units. Oligosaccharides contain from 2 up to 10 sugar units. A polysaccharide is composed of over 10 sugar units.
Mild acid hydrolysis will convert both disaccharides and polysaccharides to monosaccharides.
A monosaccharide (simple sugar) can not be converted to smaller sugar units by hydrolysis in dilute acid.
Monosaccharides are the simplest carbohydrates (simple sugars) which are not cleaved by hydrolysis to smaller carbohydrates. They are characterized by the general formula (CH2O)n, where n is any integer from 3 - 7 (3 to 7 carbons in length).
Monosaccharides are name based on either functional group or number of carbon atoms. A monosaccharide with a ketone group is referred to as a ketose. A monosaccharide with an aldehyde group is referred to as an aldose. A 3 carbon sugar is a triose, a 4 carbon sugar is a tetrose, and so on. Combining these designates such sugars as an aldotetrose or a ketopentose. For example, an aldotetrose is a four-carbon sugar that contains an aldehyde functional group.
The following table indicates the designation of a monosaccharide based on the number of carbon atoms in the molecule and functional group.
| # of carbon atoms | Aldose | Ketose |
| 3 | aldotriose | ketotriose |
| 4 | aldotetrose | ketotetrose |
| 5 | aldopentose | ketopentose |
| 6 | aldohexose | ketohexose |
In addition to these names each of the simple sugars has
a common name. Glyceraldehyde is an aldotriose. Glucose is an aldohexose.
Fructose is a ketohexose. Galactose is an aldohexose. Ribose is an aldopentose
Stereoisomerism
Isomers are compounds with identical molecular formulas.
Isomers can be categorized into the two different groups of constitutional
isomers or stereoisomers.
Constitutional isomers have the same molecular formula but a different molecular framework (different bonding constitution). Because constitutional isomers have different bonding constitutions, they are different molecules. This means that constitutional isomers have different physical and chemical properties. Ethanol CH3CH2OH and dimethyl ether CH3OCH3 are constitutional isomers. Both have the same molecular formula (C2H6O) but differ in how the atoms are connected.
Stereoisomers are molecules containing the same atoms bonded identically but the bonded atoms are oriented differently in space. That is to say, they have identical bonding constitutions but differ in how the atoms are oriented in the space around the atoms to which they are bonded.
Stereoisomers can be further separated into the two categories of diastereomers and enantiomers. One type of diastereomers (or geometric stereoisomers) differ by "cis" and "trans" orientations.
Enantiomers are a class of stereoisomers related like an object and its mirror image. Enantiomers differ in their "handedness" as the left hand and right hand are related. Enantiomers are a pair of mirror image molecules that can not be superimposed on each other. Superimposed suggests that two mirror image molecules can be mentally merged into one object as they are brought together.
There are two prominent "handed" biologically important molecules. The D- sugars and L- amino acids. The designations of D- and L- refer to how the pair of enantiomers differ in their bonding configurations. In biochemistry, D is a symbol used as a prefix to indicate the spatial configuration of certain organic compounds with asymmetric carbon atoms. It is used if an organic compound has a configuration about an asymmetric carbon atom (chiral center) analogous to that of D-glyceraldehyde (the arbitrarily chosen standard), in which the hydroxy (OH) functional group is on the right side of the asymmetric carbon atom.
The term "chirality" refers to the "handedness" of a molecule. Chiral molecules have a chiral center and these pair of molecules can not be superimposed. A chiral center is an atom with four different substituents. A carbon atom that has four different groups bonded to it is called asymmetric carbon or a chiral carbon. On the other hand, (humor!) achiral molecules (molecules "without handedness") can be superimposed.
Enantiomers are identical in most physical and chemical properties such as: melting point, boiling point, density, and chemical reactions typical for the functional groups present in the molecule.
However, there are two physical properties which permit
discernment of chirality:
1. Chiral molecules differ in their interaction with
plane polarized light. (Chiral molecules are sometimes called optical isomers.)
A polarimeter is an instrument that allows plane polarized light to pass
through aqueous solution of the molecule. The (+) isomer rotates plane
polarized light clockwise. The (-) isomer rotates plane polarized light
counterclockwise. Achiral molecules do not rotate polarized light in either
direction. Racemic mixtures contain equal mix of (+) and (-) isomers. Racemic
mixtures show NO rotation of polarized light.
2. Chiral also molecules differ in their interaction with other chiral compounds. Chiral molecules specifically recognize other chiral molecules. For example, L-amino acid protein enzyme (chiral molecule)
How many stereoisomers can a molecule have?
The number of possible stereoisomers depends upon the
number of chiral centers in the molecule. Van't Hoffs rule states: number
of stereoisomers = 2n , where n = number of chiral centers.
For example, a molecule with 2 chiral centers can have 4 stereoisomers.
Fischer projections are a
standard method for depicting the three-dimensional arrangement of atoms
on a page.
1. Tetrahedral carbon atoms are represented by two crossed
lines.
2. The horizontal lines represent bonds coming out of
the page.
3. The vertical lines represent bonds going into the
page.
4. Carbonyl carbon is place at or near the top in Fischer
projections.
5. Fischer projections can be rotated 180o
without changing their meaning, but not by 90o or 270o.
6. Carbohydrates with more than one stereogenic center
are shown by stacking the centers on top of one another, with the carbonyl
carbon again placed at or near the top. D-sugars have the stereogenic carbon
farthest from the carbonyl with the hydroxyl group written on the right
of the molecule.

Important Monosaccharides
D-Glyceraldehyde an aldotriose is the simplest carbohydrate.
It has one stereogenic center. It is a sweet colorless crystalline solid,
C3H6O3, that is an intermediate compound
in carbohydrate metabolism. D-glyceraldehyde is the arbitrarily chosen
standard for the assignment of the D configuration. In a D sugar, the hydroxy
functional group is on the right side of the asymmetric carbon atom. D-glyceraldehyde
(D for dextrorotatory) rotates light to the right.
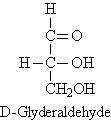
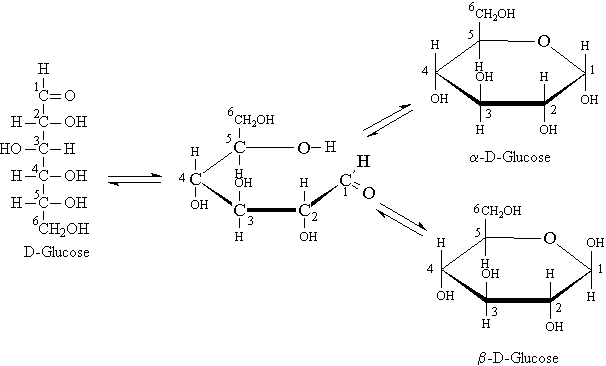
D-Glucose is an aldohexose with four stereogenic centers stacked on top of one another. It is also referred to as dextrose, grape sugar, or blood sugar. It has the empirical formula C6H12O6 . This carbohydrate occurs in the sap of most plants and in the juice of grapes and other fruits. Glucose can be obtained by hydrolysis of a variety of carbohydrates, e.g., milk and cane sugars, maltose, cellulose, or glycogen, but it is usually manufactured by hydrolysis of cornstarch with steam and dilute acid; the corn syrup thus obtained contains also some dextrins and maltose. Glucose tastes only about three-fourths as sweet as table sugar (sucrose). The presence of glucose can be detected by use of Fehlingís solution; various modifications of this test are used to detect glucose in urine, which may be a symptom of diabetes.
In actuality the open-chain form of glucose is present in very small concentrations in aqueous solutions or in living cells. It exists predominantly in either of the two cyclic forms of a-D-glucose or b-D-glucose. The hydroxyl group at C-5 reacts with the carbonyl group at C-1 to produce either of the two cyclic forms via the formation of a cyclic intramolecular hemiacetal.
Recall that hemiacetals are formed when the oxygen of a hydroxy group bonds with the carbonyl carbon of either an aldehyde or ketone.
These cyclic forms are enantiomeric pairs due to the fact that a new chiral carbon is created at C-1 in the cyclization process.
Cyclic hemiacetals are formed if both the hydroxyl and the carbonyl group are in the same molecule by an intramolecular nucleophilic addition. Five and six-membered cyclic hemiacetals are particularly stable and many carbohydrates therefore exist in equilibrium between open-chain and cyclic forms.
Pyranose is the six-membered cyclic hemiacetal formed from aldohexoses. (The name comes from the six-membered cyclic ether pyran.) Furanose is the five-membered cyclic hemiacetal formed by the ketohexose fructose. (The name comes comes from the five-membered cyclic ether furan.)
Pyranose and furanose rings can be represented by Haworth projections. Haworth projections are planar representations of the furanose and pyranose forms of carbohydrates. These type projections allow the cis-trans relationships among hydroxyl groups to be seen. In which the hemiacetal ring is drawn as if it were flat and is viewed edge-on with the oxygen atom at the upper right.
The relationship between a Fischer projection and a Haworth projection is that the group on the right in a Fischer projection is down in the Haworth projection. The group on the left in a Fischer projection is up in a Haworth projection. For D-sugars, the terminal -CH2OH group is always up in Haworth projections, whereas for L-sugars the terminal -CH2OH group is down.
Glucose exists in aqueous solution primarily as the six-membered pyranose form resulting from intramolecular nucleophilic addition of the -OH group at C5 to the C1 carbonyl group.
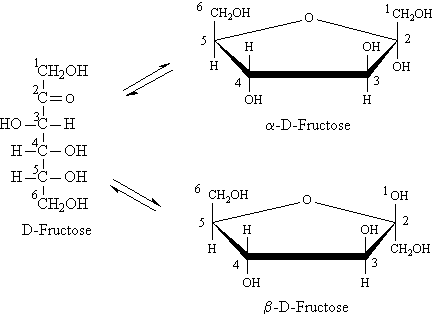
D-Fructose (levulose or fruit sugar) is the sweetest of all sugars. It is found in honey, corn syrup, and in the fruit and other parts of plants. It is much sweeter than sucrose (cane sugar). It is a ketohexose. Glucose and fructose are formed in equal amounts when sucrose is hydrolyzed by the enzyme invertase or by heating with dilute acid; the resulting equimolar mixture of fructose and glucose, called invert sugar, is the major component of honey. Fructose is a reducing sugar.
Fructose exists to the extent of about 80% in the pyranose
form and about 20% as the five-membered furanose form resulting from addition
of the -OH group at C5 to the C2 carbonyl group.
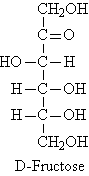 |
 |
D-Galactose is found in the biological system as a component of the disaccharide lactose, or milk sugar.

Ribose is a pentose sugar occurring as a component of riboflavin, nucleotides, and nucleic acids.
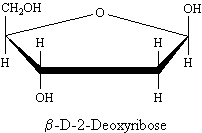
DNA, the molecule that carries the genetic information of the cell, contains 2-deoxyribose. The hydroxy group has been replaced by a hydrogen at carbon number 2, hence the designation of "2-deoxy."
Reducing sugars
Early biochemists devised analytical methods for the
detection and quantification of sugars. Some of these tests (e.g., Benedictís
Test or Fehling's reagent) were based on the aldehyde or ketone groups
in the sugar structures. Sometimes the test gave a color change as a metal
ion was reduced to the metal itself or to an ion of lower oxidation state.
In other words, the reagent oxidized the sugar while the sugar reduced
the oxidation state of the ions.
A reducing sugar is any sugar which reacts in basic Cu2+ solution to yield Cu2O precipitate (Benedictís Test). That is, they are sugars that contain aldehyde groups that can be oxidized to carboxylic acids. All monosaccharides are reducing sugars. All the common disaccharides, except sucrose, are reducing sugars. Lactose, maltose, cellobiose are reducing sugars. Sucrose is not a reducing sugar. Polysaccharides are not reducing sugars. A sugar must exist as the linear form in solution to be a reducing sugar.
Disaccharides
Oligosaccharides are formed by joining two to
ten monosaccharides.
Aldehydes react with alcohols to form hemiacetals. The hemiacetal can react further to yield an acetal.

Sugars undergo the same type of reaction to yield a glycoside.
Disaccharides are the most common oligosaccharide. These sugars are produced when two monosaccharides are linked by an "oxygen bridge" called an O-glycosidic bond.
Maltose is formed from two a-D-glucose molecules. It is a disaccharide linked by an a (1à4) glyclosidic bond. The # 1 carbon of one molecule is bonded to the #4 carbon of the other molecule. Maltose is a reducing sugar.

Lactose is formed from one galactose and one glucose molecule. It is a disaccharide linked by an b (1à4) glycosidic bond.
Sucrose is formed from one glucose and one fructose molecule. It is a disaccharide linked by an a,b (1à 2) glycosidic bond.
Polysaccharides are extended polymers of monosaccharide
units joined by O-glycosidic linkages.
Some roles of polysaccharides:
1. Energy storage.
2. Insoluble polysaccharides can serve as structural
and protective elements in cell walls of bacteria and plants and in connective
tissue and cell coats of animals.
3. Polysaccharides can lubricate skeletal joints and
provide adhesion between cells.
4. Complex sugar chains attached to lipids and proteins
can act as signals that determine the intracellular location or the metabolic
fate of these glycoconjugates.
Starch is a heterogeneous material composed other the glucose polymer amylose and amylopectin. Upper MW limit about 500,000. 20% of plant starch. Up to 80% in plants such as corn. Helical coil secondary structure. Less soluble since hydrogen bonds are intramolecular.
Amylose
The inner portion of a starch granule, consisting of
relatively soluble polysaccharides having an unbranched, linear, or spiral
structure.
Amylopectin
The outer portion of a starch granule consisting of insoluble,
highly branched polysaccharides of high molecular weight. Upper MW limit
about 1 million. 80% of plant starch. Branched, extended structure better
for storage/retrieval.
Glycogen is a polysaccharide that is the main form of carbohydrate storage in animals and occurs primarily in the liver and muscle tissue. It is readily converted to glucose as needed by the body to satisfy its energy needs. Also called animal starch. Branched, extended structure better for storage/retrieval.
Cellulose is the most abundant polysaccharide, indeed the most abundant organic molecule in the world. Plant structural sugar. Straight fiber-like secondary structure. Each residue is turned 180 degrees relative to the preceding residue.