

Amides are another group of nitrogen-containing organic compounds.

Structure and Physical Properties
Amines can be thought of as organic derivatives of ammonia.
Just as ammonia is basic, so are amines.

Amines with fewer than 5 carbons are miscible with water.
Amines are classified based on the number of organic substituents on nitrogen. This is analogous to carbon but the atom of reference is now nitrogen.
Primary (1o): One substituent - CH3NH2
Secondary (2o): Two substituents - (CH3)2NH
Tertiary (3o): Three substituents - (CH3)3N
Quaternary ammonium salts - (CH3)4N1+
Primary and secondary form hydrogen bonds. Low-molecular-weight amines have fish-like odors.
Amine Nomenclature
The Chemical Abstracts or CA system drops the final-e
of the name of the parent hydrocarbon, and adds the suffix -amine.
Methanamine CH3-NH2
Ethanamine CH3-CH2-NH2
Propanamine CH3-CH2-CH2-NH2
For secondary or tertiary amines the prefix N-alkyl is
added to the name of the parent compound.
N-Methylethanamine CH3-NH-CH2-CH3
N,N-Dimethylmethanamine CH3-N(CH3)2
![]()
Aniline (or benzenamine) is an aromatic amine. Ph-NH2
Common amines.
| Primary: One substituent
CH3NH2 Methanamine
|
Secondary: Two substituents
(CH3)2NH N-methylmethanamine
|
| Tertiary: Three substituents
(CH3)3N N,N-dimethylmethanamine
|
Quaternary ammonium salts
(CH3)4N1+X1- Tetramethylammonium iodide
|
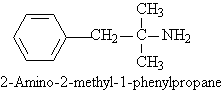
Medically Important Amines

One of the components of fen-phen, is 2-amino-2-methyl-1-phenylpropane.

Preparation of Amines
1. Reduction of amides
[H]
RCONH2 -----> RCH2NH2
2. Reduction of aryl nitro to aniline.
[H]
Ph-NO2 -----> Ph-NH2
Basicity of Amines
Amine chemistry is dominated by the lone pair on the
nitrogen atom. Amines behave as weak bases when dissolved in
water producing an alkylammonium ion and the hydroxide ion.
RNH2 + H2O <====> RNH31+ + OH1-

Neutralization of Amines
Amines react with acids to form alkylammonium salts.
Amine + Acid --> Alkylammonium salt
RNH2 + HCl ---> RNH31+ + Cl1-

Quaternary Ammonium Salts
Quaternary ammonium salts are ammonium slats that have
four organic groups bonded to the nitrogen.
R4N+X- [Where R = any alkyl or aryl group and X- = a halide ion, most often chloride, Cl-]
Quaternary ammonium salts that have very long carbon chains, sometimes called "quats," are used as disinfectants and antiseptics because they have detergent activity.
Benzalkonium chloride (ZephiranTM) is a quat found in and cetylpyridinium chloride is a component of the mouthwash Cepacol.

Dimethyl benzyl ammonium saccharinate is a quat found in Lysol spray.
Choline is an important quaternary ammonium salt in the body. It is part of the hydrophilic "head" of the membrane phospholipid lecithin. Choline is also a precursor for the synthesis of the neurotransmitter acetylcholine.

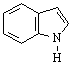
Heterocyclic Amines
Heterocyclic amines are compounds in which the nitrogen
atom occurs as part of a ring. Each ring has its own parent name with the
nitrogen as the number 1 position.
| Pyrrole |
Pyridine |
Pyrimidine |
Purine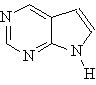
|
Indole
|
Alkaloids are naturally occurring compounds with one or
more nitrogen-containing heterocyclic rings.
Cocaine; Nicotine; Quinine; Morphine; Heroin; LSD
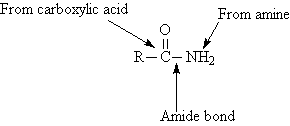
Amides
Amides are derivatives of carboxylic acids. The amide
group is composed of two portions. The bond between the carbonyl carbon
and the nitrogen of the amine or ammonia is called the amide bond.
Structure and Physical Properties of Amides
Most are solid at room temp. Amides are NOT bases (proton
acceptors).
Amide Nomenclature
Remove the -ic of the parent acid ending and replace
it with -amide.
Ethanoic acid ---> Ethanamide
Propanoic acid ---> Propanamide
Substituents on the nitrogen are placed as prefixes and
are indicated by N- followed by the name of the substituent. There are
no spaces between the prefix and the amide name.
N-Methylpropanamide
N-Propylhexanamide
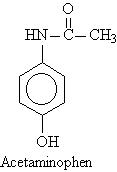
Preparation of Amides
Amides are most often prepared by reaction of an acid
chloride with an amine. Ammonia, mono- and disubstituted amines all undergo
the reaction.
Acid + PCl5 ---> Acid chloride
Acid chloride + Ammonia or Amine ----> Amide
Hydrolysis of Amides
Amides undergo hydrolysis to yield carboxylic acids plus
amine on heating in either aqueous acid or aqueous base. The conditions
required for amide hydrolysis are more severe than those required for the
hydrolysis of acid chlorides or esters, but the mechanisms are similar.
Preview Of Amino Acids, Proteins, And Protein Synthesis
Proteins are polymers of amino acids. As the name suggests, amino acids have two essential functional groups, an amino group and a carboxyl group.

In the cell the amino group is protonated and the carboxyl group is ionized to the carboxylate anion. This is shown in the following way.

The amide bond that forms between the carboxyl group of one amino acid and the amino group of another is called the peptide bond.