Why are there so many carbon containing compounds?
1. Carbon can form stable, covalent
bonds with other carbon atoms (catenate).
2. Carbon can form stable bonds with
other elements.
3. Carbon can form double and triple
bonds with other carbon atoms or other elements.
4. Carbon can bond with itself either
as straight chains or cyclic compounds with branching possible in either
case.
Two different organic compounds can also have the same number of carbon atoms. These are called isomers. Isomers are molecules that have the same molecular formulas but different structures and thus different physical and chemical characteristics. An example of isomerism is butane and 2-methylpropane. Both have the same molecular formula, C4H10, but differing physical and chemical properties.
Contrasts Between Organic and Inorganic Molecules
Carbon bonds are almost always covalent. Covalent compounds
are often referred to as molecules.
Most bonds between a metal and a nonmetal are ionic.
Many inorganic compounds are ionic compounds.
Ionic bonds:
1. result from the transfer of atoms
and not sharing.
2. are electrostatic.
3. result with formation of large
lattice crystals of cations and anions.
4. often dissolve in water to give
electrolytic solutions (i.e., they conduct electricity).
Classification of Organic Compounds
All organic compounds be generally classified as hydrocarbons
(molecules containing only hydrogen and carbon) or substituted hydrocarbons
(molecules having one or more hydrogen atoms replaced by another atom or
group of atoms).
Hydrocarbons can be further subdivided into Aliphatic (Greek aleiphat "fat") and Aromatic.
Aliphatic can be divided into alkanes, alkenes, and alkynes.
Alkanes are saturated hydrocarbons. Saturated means that the hydrocarbon has only single bonds and that the hydrocarbon contains the maximum number of hydrogen atoms for each carbon atom. Unsaturated hydrocarbons contain multiple bonding and contain less than the maximum number of hydrogens per carbon.
Ethane, C2H6, is an alkane and is an example of a saturated hydrocarbon.

Ethene, C2H4, is an alkene and is an example of unsaturated hydrocarbon.

Ethyne, C2H2, is an alkyne and is also an example of unsaturated hydrocarbon.
Aromatic compounds are unsaturated hydrocarbons and contain a benzene ring.
| Functional Group | Name | Family of Organic Compounds | Example |
|
|
carbon-carbon double bond | Alkene | 1-Butene |
|
|
carbon-carbon triple bond | Alkyne | 1-Butyne |
|
|
Benzene ring | Aromatic | Toluene |
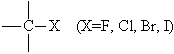 |
Halogen atom (-X) | Alkyl halide | Chloromethane
CH3Cl |
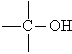 |
Hydroxyl group (-OH) | Alcohol | Ethanol
CH3CH2OH |
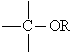 |
Alkoxy group (-OR) | Ether | Dimethyl ether
CH3-O-CH3 |
|
|
Carbonyl group | Aldehyde or ketone | Ethanal
|
|
|
Carboxyl group | Carboxylic acids | Acetic acid |
|
|
Acyl group | Carboxylic acid derivatives | Methyl acetate |
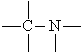 |
Amino group | Amines | Methyl amine |
Alkanes
Alkanes are saturated hydrocarbons. This means that contain
only carbon and hydrogen atoms bonded by single bonds only.
The general formula for an alkane is CnH2n+2.
In this formula, n, is the number of carbon atoms in the molecule.
The molecular formula is the actual ratio of atoms to
one another in a molecule.
A structural formula represents a
structure and emphasizes the bond connection between atoms.
A condensed formula is a simplification
of the structural formula.
A line formula is a simplified representation
of a structural formula in which many of the C-H bonds are not shown.
In a line
formula, the carbons are understood to exist at the vertices of each of
the angles and the number of
hydrogens
necessary are also understood, though not written.
|
|
Formula |
Formula |
Formula |
Formula |
| Propane | C3H8 | 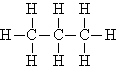 |
CH3CH2CH3 |
|
| Butane | C4H10 | 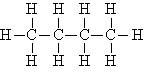 |
CH3CH2CH2CH3 |
Names and Formulas of the First Ten Straight-Chain Alkanes & Alkyl Groups
|
|
Formula |
|
group |
|
|
|
|
CH4 |
|
CH3- |
|
|
|
CH3-CH3 |
|
CH3-CH2- |
|
|
|
CH3-CH2-CH3 |
|
CH3-CH2-CH2- |
|
|
|
CH3-CH2-CH2-CH3 |
|
CH3CH2CH2CH2- |
|
|
|
CH3CH2CH2CH2CH3
or CH3-(CH2)3-CH3 |
|
CH3-(CH2)3-CH2- |
|
|
|
CH3-(CH2)4-CH3 |
|
CH3-(CH2)4-CH2- |
|
|
|
CH3-(CH2)5-CH3 |
|
CH3-(CH2)5-CH2- |
|
|
|
CH3-(CH2)6-CH3 |
|
CH3-(CH2)6-CH2- |
|
|
|
CH3-(CH2)7-CH3 |
|
CH3-(CH2)7-CH2- |
|
|
|
CH3-(CH2)8-CH3 |
|
CH3-(CH2)8-CH2- |
Hydrocarbon carbon bonding characteristics:
Carbon always forms 4 bonds with no lone pair electrons
on the carbon atom. (One exception of this is carbon monoxide.)
Single bonded carbon atoms have bond angles of 109.5o.
Alkanes are composed of carbon atoms that have tetrahedral geometry.
Any compound with carbon having four single bonds will have a tetrahedral
shape about any of the single bonded carbon atoms, with the bonded atoms
at the vertices of a tetrahedron.
Hydrocarbon physical characteristics:
Hydrocarbons are nonpolar. They are immiscible with water.
They have relatively low melting points and boiling points.
The first four alkanes (methane – butane) are all gases at room temperature.
Hydrocarbons are generally less dense than water.
Compounds that differ only in the number of -CH2-
groups inserted in the carbon chain form a family group called a homologous
series.
Members of this family are called homologs.
Members of a homologous series are very similar in their
chemical reactivity.
As more carbons are added and the size of the molecule
increases, they exhibit gradually changing physical properties.
As number of carbon atoms increases (increase in molecular
weight) the melting points and boiling points.
Except for the very small alkanes, the boiling point
rises 20 to 30 degrees for each carbon that is added to the chain.
The first four alkanes (i.e. with fewer than 5 carbons)
are gases at room temp.
Alkyl groups
When a hydrogen is removed from a hydrocarbon, the resulting
fragment is called an alkyl group. The name of the alkyl group is derived
from the alkane by removing the –ane and replacing with –yl. An alkyl group
can be represented by "R" in chemical structures.
| Alkane | Condensed Lewis
Structure |
Alkyl
group |
R- |
| Methane | CH4 | methyl | CH3- |
| Ethane | CH3-CH3 | ethyl | CH3CH2- |
| Propane | CH3-CH2-CH3 | propyl | CH3CH2CH2- |
| Butane | CH3-CH2-CH2-CH3 | butyl | CH3CH2CH2CH2- |
| Pentane | CH3CH2CH2CH2CH3 | pentyl | CH3CH2CH2CH2CH2- |
Carbon atoms are classified by the number of other carbon
atoms bonded to it.
A primary carbon (1o) is bonded to one carbon.
A secondary carbon (2o) is bonded to two carbons.
A tertiary carbon (3o) is bonded to three
carbons.
A quaternary carbon (4o) is bonded to four
carbons.
Alkyl groups are classified according to the number of carbons attached to the carbon that joins the alkyl group to the molecule.
| primary
alkyl group |
secondary
alkyl group |
tertiary
alkyl group |
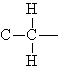 |
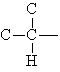 |
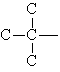 |
Hydrogen atoms are classified according to the type of
carbon to which they are attached and are given equivalent names.
Primary = connected to a primary carbon.
Secondary = connected to a secondary carbon.
Tertiary = connected to a tertiary carbon.
Remove a primary hydrogen from propane and the resulting
alkyl group is the normal propyl group or n-propyl group.
n-propyl CH3CH2CH2-
Remove a secondary hydrogen from propane and the resulting
alkyl group is a isopropyl group.
isopropyl (CH3)2CH-
The alkyl groups the butanes, C4H10, are n-butyl, sec-butyl, iso-butyl, and tert-butyl.
Nomenclature of Alkanes
A chemical name has three parts in the IUPAC system:
Prefix, Parent, Suffix.
Prefix gives the location.
Parent or stem tells how many carbons.
Suffix identifies the functional group.
I.U.P.A.C. Nomenclature of Alkanes
1. Determine the parent compound find the longest
continuous chain of carbon atoms. If two different chains of equal length
are present, choose the one with the larger number of branch points.
2. Name and number the atoms. The stem name is derived from the parent hydrocarbon.
3. Number the parent chain to give the lowest number to the carbon bonded to the first group encountered on the parent chain.
4. If the same substituent occurs more than once, the number of each carbon of the parent alkane to which it is attached is given, and the number of substituent groups involved is indicated by prefix such as di-, tri, tetra-, and so on.
5. Name the compound, beginning with the side chains
in alphabetical order and ending with the name of the parent compound.
Follow these rules:
a. Always use commas between
numbers.
b. Always use hyphens
between numbers and words.
c. Do not leave spaces
in the name.
d. When alphabetizing
substituent groups, ignore the prefixes, di-, tri-, and so on.
e. Use the lowest possible
numbers.
Constitutional isomers
Isomers are compounds with identical molecular formulas
but which differ in the atomic arrangements. These compounds differ
in shape and properties and are actually different compounds. Constitutional
isomers are molecules having the same molecular formula but different arrangement
of atoms (e.g., n-butane and 2-methylpropane). Geometric isomers
are molecules which have the same molecular formula but differing spatial
arrangement of atoms due to absence of free rotation.
There are 2 butane, C4H10, isomers.
There are 3 pentane, C5H12, isomers.
There are 4 hexane, C6H14, isomers.
There are 9 heptane, C7H16, isomers.
There are 18 octane, C8H18, isomers.
There are 35 nonane, C9H20, isomers.
There are 75 decane, C10H22, isomers.
Cycloalkanes
The cycloalkanes have the general formula of CnH2n.
|
Cyclopropane C3H6 |
Cyclobutane C4H8 |
Cyclopentane C5H10 |
Cyclohexane C6H12 |
Cycloheptane C7H14 |
I.U.P.A.C. Nomenclature of Cycloalkanes
1. Count the number of carbon atoms in the ring and the
number in the largest substituent. If the number of carbon atoms in the
ring is equal to or greater than the number in the largest substituent,
the compound in named as an alkyl-substituted cycloalkane. If the number
of carbon atoms in the largest substituent is greater than the number in
the ring, the compound is named as a cycloalkyl-substituted alkane.
2. For alkyl-substituted cycloalkanes, start at a point
of attachment and number the substituents on the ring so as to arrive at
the lowest sum.
a. Number substituents alphabetically.
b. Halogen are treated exactly like
alkyl groups.
3. Use hyphen after cis or trans.
Cis-Trans Isomerism in Cycloalkanes
Rotation around the bonds in a cyclic structure is limited.
The formation of cis-trans isomers or geometric isomers, is a consequence
of the absence of the free rotation. Geometric isomers are a type of stereoisomer.
The term "cis" is derived from Latin and means "on the same side."
The term "trans" is also derived from Latin and means "across from."
Conformations of Alkanes and Cycloalkanes
Because there is free rotation around a carbon-carbon
single bond, even a very simple alkane like ethane, can exist in an unlimited
number of forms. Cycloalkanes (except cyclopropane) also exist in
different conformations. Cyclohexane exist in a "chair conformation"
(the most energetically favorable) or a "boat conformation."
The hydrogen atoms which lie above or below the ring are said to be axial.
Those that lie roughly in the plane of the ring are called equatorial.
When the chair form "flips", the six equatorial hydrogens become axial
and vice versa for the six axial.
The axial hydrogens are actually quite crowded. If one of them is replaced by a larger group, even one as small as a methyl group, the chair form in which the larger substituent is equatorial is strongly favored. This minimizes the crowding, resulting in a more stable structure.
The boat conformation is less stable than the chair because the hydrogen atoms are not perfectly staggered.
Reactions of Alkanes and Cycloalkanes
Combustion
Hydrocarbons react with an excess of oxygen to yield
carbon dioxide and water.
CH4 + 2 O2 -->
CO2 + 2 H2O + Heat
2 C2H6 + 7 O2
--> 4 CO2 + 6 H2O +
Heat
If alkanes are burned with insufficient oxygen then carbon
monoxide is formed instead of carbon dioxide.
2 CH4 + 3 O2
--> 2 CO + 4 H2O + Heat (?)
2 C2H6 + 5 O2
--> 4 CO + 6 H2O
Halogenation
Halogenation is a substitution reaction. Substitution
reactions involve replacement of an atom with another atom or group of
atoms. In halogenation, hydrogens in the hydrocarbon are substituted
with a halogen (Cl or Br). The product of a halogenation is an alkyl
halide (RX) and hydrogen halide.
light or heat (200-400oC)
R-H + X2
-------------------------> R-X + HCl
X2 represents either Cl2 or Br2.
[Iodine, I2, does not work.]
Tertiary hydrogens (3o ) are replaced more
rapidly than secondary ( 2o) which react faster than primary
(1o ).
If the halogenation reaction is allowed to continue, the
alkyl halide can react with halogen atoms and more than one product can
be formed, resulting in a mixture of products.
CH4 + Br2 -->
CH3Br + HBr
CH3Br + Br2
--> CH2Br2 + HBr
CH2Br2 + Br2
--> CHBr3 + HBr
CHBr3 + Br2
--> CBr4 + HBr
n-propane + Cl2/light ---> 1-chloropropane
+ 2-chloropropane
45%
55%
n-butane + Cl2/light ---> 1-chlorobutane +
2-chlorobutane
28%
72%
isobutane + Cl2/light ---> 1-chloro-2-methylpropane
+ 2-chloro-2-methylpropane
64%
Chlorination gives mixtures in which no isomer greatly predominates; in bromination, by contrast, one isomer may predominate to such an extent as to be almost the only product, making up 97-99% of the total mixture.
Bromination of the same alkanes above results with the more substituted carbon being the site of bromination with only trace amounts of the other possibilities.