Aldehydes and ketones are polar compounds.
The carbonyl carbon is partially positive (?+) and
the carbonyl oxygen is partially negative (?-).

Structure and Physical Properties
Aldehydes and ketones are polar compounds.
The carbonyl carbon is partially positive (?+)
and
the carbonyl oxygen is partially
negative (?-).

Aldehydes and ketones have higher melting and boiling points than hydrocarbons of equivalent molecular weight. This is due to the attraction between molecule due to the polarity of the carbonyl group. This type of attraction is referred to as dipole-dipole forces of attraction.
IUPAC Rules for Naming Aldehydes
1. Determine the parent hydrocarbon.
2. Replace -e with ľal.
3. Number the carbon chain with the carbonyl group as
number one.
IUPAC and Common names of Aldehydes
| Methanal (formaldehyde) |
Ethanal (acetaldehyde) |
| Propanal (propionaldehyde) |
Butanal (butyraldehyde) |
| Pentanal (valeraldehyde) |
2-Methylpropanal  |
The common names of the aldehydes are derived from the
same Latin roots as the corresponding carboxylic acids. In the common system
of nomenclature, substituted aldehydes are named as derivative of the straight-chain
parent compound.
![]()
Greek letters are used to indicate the position of the
substituents.

2-Phenylbutanal = a-phenylbutyraldehyde

3-Methylpentanal = b-methylvaleraldehyde
IUPAC Rules for Naming Ketones
1. Determine parent hydrocarbon.
2. Replace -e with -one.
3. Carbonyl carbon is numbered as lowest possible value.
| Propanone (acetone) |
Butanone (methyl ethyl ketone) |
| 2-Pentanone |
3-Pentanone |
| Acetophenone |
Benzophenone |
Common Aldehydes and Ketones
(The following information was obtained at: http://www.bartleby.com.)
Formaldehyde (methanal) is a colorless gaseous compound,
HCHO, the simplest aldehyde, used for manufacturing melamine and phenolic
resins, fertilizers, dyes, and embalming fluids and in aqueous solution
as a preservative and disinfectant.
Acetaldehyde (ethanal) is a colorless, flammable liquid, C2H4O, used to manufacture acetic acid, perfumes, and drugs. Also called aldehyde.
Acetone (propanone) is a colorless, volatile, extremely flammable liquid ketone, CH3COCH3, widely used as an organic solvent.
Benzaldehyde is a normally colorless aromatic oil, C6H5CHO,
obtained naturally, as from the bitter almond, or made synthetically and
used in perfumes and as a solvent and a flavoring.

Reactions of Aldehydes and Ketones
Preparation of Aldehydes and Ketones
|
1o alcohol ------> Aldehyde |
2o alcohol ------> Ketone |
|
3o alcohol ------> NR |
Methanol -----> Methanal |
Oxidation Reactions
Aldehydes are easily oxidized to carboxylic acids, whereas
ketones do not generally undergo further oxidation.


Tollen's Test for Aldehydes
Aldehyde + AgNO3 + NaOH + NH3 --->
RCO21-Na1+ + Ag
Benedict's or Fehling's Test for Aldehydes
Aldehyde + [Cu2+complex] --> RCO2H
+ Cu2O
Blue
Brick-red
Reduction Reactions
Hydrogenation
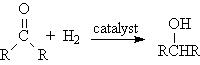
Addition Reactions
If a carbonyl compound is treated with excess alcohol
in the presence of a trace of acid, aldehydes will give an acetal product,
whereas ketones will give a ketal product.


All molecule of this general type will be classified as acetals.
Keto-enol tautomerism
Carbonyl compounds with
one or more hydrogens on their a-carbons
rapidly interconvert with their corresponding enols.
Aldol Condensation
The aldol condensation is a reaction in which aldehydes
and ketones react to form larger molecules. A new carbon-carbon bond is
formed.
In this generalized reaction the a-carbon of one aldehyde forms a bond with the carbonyl carbon of the other aldehyde.

2 Acetaldehyde +
Sodium ethoxide ---> Aldol (a
b-hydroxy
aldehyde)
Suggested Problems
14.23
14.30
14.37 a,b,c,d
14.41